- LOGIN
- MemberShip
- 2025-12-24 05:40:00
- Company
- GM Lee Seung-woo to leave Gilead Sciences Korea
- by Eo, Yun-Ho Jan 16, 2023 06:02am
- General Manager Paul Seung-woo Lee Paul Seung-woo Lee (65), who has served as the General Manager of Gilead Sciences Korea and led the company since its establishment, will be leaving the company. According to industry sources, Lee has recently expressed his intent to resign upon the expiry of his term to the company. Accordingly, Gilead is currently in the process of hiring Lee’s successor. Lee is known to have decided to retire with his resignation. Lee is regarded as a symbolic figure as CEO of multinational pharmaceutical companies in Korea’s pharmaceutical industry. After graduating from the University of Alberta and receiving an MBA from Columbia Business School, Lee worked at Johnson & Johnson and Korea Research-based Pharma Industry Association. In 1996, after being appointed as Managing Director of MSD Korea, Lee served as CEO of Korean subsidiaries of various multinational companies including AstraZeneca and Wyeth (currently Pfizer). In 2011, Lee was appointed the founding head of Gilead’s Korean subsidiary and has held the position ever since. In other words, Lee has served as CEO of multinational companies for over 25 years. Considerable changes are expected in the organization as other founding members of the company including Senior Director Yeonsim Jeong are expected to retire soon in addition to GM Lee. Also, a fierce competition is expected in the hiring of CEO Lee’s replacement as a large number of applicants applied for the position. Also, the company is also currently in the process of hiring other executive-level personnel for MA, marketing, etc.
- Company
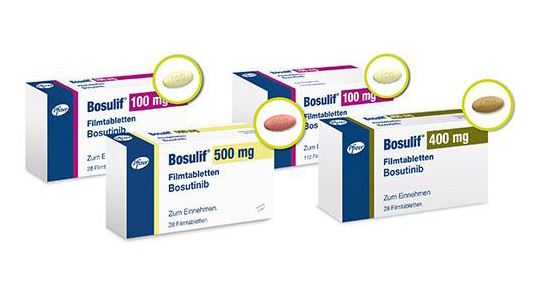
- Bosulif approved in Korea 11 years after US approval
- by Jan 16, 2023 06:02am
- Bosulif(bosutinib.(Pic=Pfizer)The Ministry of Food and Drug Safety approved the new drug for chronic phase Philadelphia chromosome-positive chronic myelogenous leukemia (Ph+ CML), ‘Bosulif (bosutinib)’ in Korea. The drug was first approved in the US in 2012. With the approval, the treatment options for Ph+CML increased to 6, excluding the first-generation drug. According to industry sources on the 14th, the MFDS approved 3 dosages (100·400·500mg) of Pfizer Korea’s new drug for Ph+ CML Bosulif on the 12th. The drug is now indicated for the treatment of newly diagnosed chronic Ph+ CML or chronic, accelerated, or blast-phase Ph+ CML with resistance or intolerance to prior therapy. Bosulif is a second-generation treatment that is taken orally once daily. Bosulif was first approved by the US Food and Drug Administration (FDA) in 2012 for the treatment of Ph+ CML with resistance to prior therapy. Its indication as a first-line treatment for newly diagnosed Ph+ CML has been additionally approved in the US in 2017, and in 2018 in Europe. The drug was finally approved in Korea 11 years after it was first approved by the FDA. A Pfizer Korea official said, “We received approval for Bosulif in Korea this year based upon our strategic judgment of the domestic CML treatment market.” Chronic myeloid leukemia including PH+ CML is a malignant hematologic disease that occurs when the bone marrow produces white blood cells. CML is a very slow, chronically progressing blood cancer, and over 90% of patients with CML are found with a characteristic gene abnormality in the Philadelphia (Ph) chromosome, which causes an increase in white blood cells and platelets. The Ph chromosome forms when chromosome 9 and chromosome 22 break and exchange portions, and the byproduct that appears in the process can grow cancer cells. Bosulif’s safety and efficacy were verified through a Phase III trial (NCT02130557) on the drug’s use as a first-line therapy that was conducted on patients with newly-diagnosed Ph+ CML. The major efficacy outcome measure was major molecular response (MMR) at 12 months. Results showed that MMR at 12 months was 47% in the Bosulif arm and 36% in the Glivec (imatinib) arm. MMR at 60 months was 74% in the Bosulif arm and 66% in the Glivec arm. The median time to MMR in respondents after 60 weeks of follow-up was 9.0 months in the Bosulif arm and 11.9 months in the Glivec arm. The indication for chronic, accelerated, or blast phase Ph+ CML with resistance or intolerance to prior therapy was obtained based on Phase I/II trial (NCT00261846), which evaluated the efficacy and safety of the once-daily administration of the 500mg dose of Bosulif. Its major efficacy endpoints included the rate of attaining major cytogenetic response (MCyR) by Week 24 and the duration of MCyR. 40% of patients that had previously received Glivec monotherapy reached MCyR by Week 24. The most common adverse reactions in the 814 patients that participated in the two trials were diarrhea (80%), rash (44%), nausea (44%), abdominal pain (43%), vomiting (33%), fatigue (33%), hepatic dysfunction (33%), respiratory tract infection (25%), pyrexia (24%), and headache (21%). 22% of the 268 patients that were newly diagnosed with Ph+ CML showed serious adverse reactions. Serious adverse reactions reported in >2% of patients included hepatic dysfunction (4%), pneumonia (3%), coronary artery disease (3%), and gastroenteritis (2%). In Korea, first to fourth-generation drugs are available for the treatment of Ph+ CML. Bosulif is a second-generation drug. The first-generation treatment is Novartis Korea’s Glivec, and generic versions of the drug have been released upon its patent expiry. The second-generation treatments include Novartis Korea’s ‘Tasigna (nilotinib),’ BMS Korea’s ‘Sprycel (dasatinib),’ Il-Yang Pharmaceutical’s ‘Supect Capsule (radotinib),’ etc. Otsuka Pharmaceutical Korea’s Iclusig (ponatinib)’ is a third generation treatment. As a fourth-generation treatment, there is Novartis Korea’s Scemblix (asciminib). Scemblix was approved in June last year as a treatment for adult patients with Ph+ CML in the chronic phase previously treated with two or more tyrosine kinase inhibitors (TKIs), However, the drug has not yet been applied for reimbursement in Korea.
- Company
- RET-targeted Retevmo lands in general hospitals in KOR
- by Eo, Yun-Ho Jan 13, 2023 06:02am
- The RET-targeted anticancer therapy ‘Retevmo’ may now be prescribed at general hospitals in Korea. According to industry sources, Lilly Korea’s, Lilly Korea’s Retevmo (selpercatinib) passed the drug committees of tertiary hospitals in Korea including the Samsung Medical Center, Seoul National University Hospital, and Seoul St. Mary’s Hospital. Some hospitals were found to have set local drug codes after holding emergency Drug Committee meetings. Therefore, if Retevmo is listed for reimbursement, the listing is expected to quickly lead to actual prescriptions. Retevmo, which received marketing authorization in March last year, was unable to pass CDDC review for reimbursement in May, but then passed review in November and is awaiting deliberation by the Drug Reimbursement Evaluation Committee. Retevmo is indicated for: ▲adult patients with metastatic RET fusion-positive non-small cell lung cancer (NSCLC); ▲adults and pediatric patients 12 years of age or older with advanced or metastatic RET-mutated medullary thyroid cancer who require systemic therapy; and ▲ adult patients who are refractory to radioactive iodine therapy and who have prior sorafenib and/or lenvatinib treatment, with advanced or metastatic RET-fusion benign thyroid cancer who require systemic therapy. The drug demonstrated its efficacy through the LIBRETTO-001 trial that was conducted on 702 patients with advanced or metastatic solid cancer with RET mutations. Patients with RET fusion-positive NSCLC, RET-mutated medullary thyroid cancer, and RET fusion-positive thyroid cancer patients with or without prior treatment experience were enrolled in the LIBRETTO-001 trial. The primary endpoints of the trial were the objective response rate (ORR) and duration of response (DOR) as assessed by the independent review committee. In patients with RET fusion-positive NSCLC without platinum-based treatment experience, the ORR in the Retevmo-treated group was 85%. Although the median DoR was not yet reached, 79% of the patients showed a durated response during the follow-up period (median 7.4 months). In patients with platinum-based treatment experience, the ORR was 64%, and the median DoR was 17.5 months. Professor Min-Hee Hong of Oncology at Yonsei Cancer Center said, “Lung cancer patients with RET mutations are twice more likely to experience CNS metastasis, but had to be treated with chemotherapy due to the lack of options until now, which is less effective and more prone to toxicity.” Hong added, “Retevmo demonstrated a significant response in the LIBRETTO-001 trial, as well as an 82% ORR in patients with CNS metastasis. 23% of these patients achieved complete response.”
- Company
- GC Pharma exclusively distributes and sells Baraclude
- by Kim, Jin-Gu Jan 13, 2023 06:01am
- GC Pharma announced on the 11th that it will extend the contract to sell Baraclude, a hepatitis B treatment by BMS Korea, and expand its partnership to exclusive distribution and sales. The two companies have continued their partnership related to Baraclude since September 2015. Through this contract, GC Pharma strengthened its influence through exclusive distribution and sales in the form of Co-promotion. In addition, the sales area will be expanded from the hospital and clinic level to all hospitals, including general hospitals. Baraclude is a hepatitis B treatment. With its strong virus inhibition effect and low resistance expression rate, it has topped the list of prescriptions for years since its launch in Korea in 2007.
- Company
- Baxter's core business unit spun off
- by jung, sae-im Jan 12, 2023 04:32am
- Baxter will spin off its key business unit. The industry expects the division to become independent and sell it to private equity funds. In the aftermath, the Korean branch is reducing its workforce. According to the pharmaceutical industry on the 12th, Baxter Korea recently conducted voluntary retirement (ERP) for its employees. Those who have worked for more than 10 years or were born in 1977 are eligible. The compensation condition is known as '2n+2'. This means that retirement benefits will be paid twice the number of years of service plus two months. The reduction of Baxter Korea's workforce is related to the global headquarters' decision to spin off its business units. Recently, Baxter announced that it will spin off its core business unit. The plan is to spin off 'Renal Care and Acute Therapies Global Business Units (GBUs)' and list it newly. The Xinjiang division accounts for about half of Baxter's total sales. It also includes peritoneal dialysis machines, which have the highest sales, and hemodialysis products, which are major products. The independent corporation also includes a portfolio of new products to be released in the future. The industry expects Baxter to spin off its key department into an independent corporation and then go through the sale process. It is predicted that the amount sold will be used to pay off the debt of the acquisition fund. In 2021, Baxter acquired Hilom, a digital medical device company, for $10.5 billion (about 12 trillion won) in cash. 분사되는 신장 사업부(좌)와 기존 박스터 매출(자료: 박스터) Baxter has a precedent of selling its hemophilia treatment division in 2015. The division, which includes items related to hemophilia treatments such as "Adbate," was established as an independent corporation called "Box Alta" and sold to Shire for about 32 billion dollars (about 38 trillion won) the following year. It was the largest M&A transaction in the pharmaceutical industry at the time. As the Xinjiang division is also making solid sales as a key division of Baxter, it is predicted that a "big deal" will occur. However, the decision to spin off is expected to lead to confusion in the Korean branch. An industry official said, "Baxter has built a solid position by operating the Xinjiang division for more than 60 years. However, he/she seems to have decided to spin off because he/she believes that there is not much room to expand the market, such as losing market share to competitors recently, he/she said. "The company is in a chaotic atmosphere due to issues such as manpower reduction, spin-off, and sale."
- Company
- Celltrion launches Stella PO development with U.S. Rani
- by Jan 12, 2023 04:30am
- Celltrion Research Institute is conducting research and development on pharmaceuticals.Celltrion announced on the 9th that it has signed a contract with Rani Theraputics, a bio company in San Jose, USA, to develop Stella PO. Celltrion exclusively supplies Rani Theraputics with the Stella biosimilar CT-P43, which is needed for Stella PO (RT-111) non-clinical and phase 1 clinical trials. In the future, it will have priority negotiation rights on global development and sales rights. Rani Theraputics has developed a platform technology that can use intravenous and subcutaneous injection-type protein and antibody drugs for oral use through its own oral capsule platform RaniPill capsule. Oral capsules made with RaniPill technology break down capsules in the small intestine. The drug is delivered to the small intestine through a soluble microneedle in the capsule and then transferred to the blood vessels. Although it is an oral drug, it is a platform technology designed to deliver drugs similar to injections through microneedles mounted on capsules. CT-P43's original drug, interleukin (IL)-12, 23 inhibitor Stella, is intended for two types of intravenous and subcutaneous injections. It is used for indications such as flake psoriasis, Crohn's disease, ulcerative colitis, and psoriatic arthritis. According to Johnson & Johnson's management performance, Stella is a blockbuster biopharmaceutical that recorded 9.134 billion dollars in sales in 2021. Starting with oral Stella development collaboration, Celltrion plans to expand collaboration in applying innovative drug delivery platforms not only through CT-P43 but also throughout its product pipeline. Through this, it is expected that the products currently being developed will also have differentiated competitiveness.
- Company
- 80 companies participated in the K-Cab patent dispute
- by Kim, Jin-Gu Jan 12, 2023 04:30am
- The patent dispute over HK Innoen's gastroesophageal reflux disease treatment K-Cab has expanded to the largest scale ever. A total of 80 companies challenged HK inno.N. According to the pharmaceutical industry on the 10th, a total of 80 companies have challenged K-Cab crystalline patents by the 9th. On December 24 last year, SCD filed a passive judgment on the scope of rights in the K-Cab crystalline patent for the first time. By the 9th, 80 companies challenged the same patent. These 80 companies qualified for the "first trial request," one of the generic for exclusivity requirements, by filing the same trial within 14 days of the SCD's filing of the trial. It is the largest ever based on a single item. Previously, there were large-scale patent challenges in the pharmaceutical industry, but there were 40 to 50 participating companies. However, Chong Kun-dang and Daewoong Pharmaceutical, which were active in challenging pharmaceutical bio patents, did not enter the dispute. This is because Chong Kun Dang jointly sells HK inno.N and K-Cab. Analysts say that Daewoong Pharmaceutical is selling Fexuclu, a competitive drug of K-Cab. During this period, 247 documents were filed with the Patent Tribunal. The figure adds to all the cases in which a generic company filed a judgment for the purpose of more than two claims. K-Cab is protected by a total of two patents. It is a substance patent that expires in August 2031, and a crystalline patent that expires in March 2036. Among them, the patents requested by generic companies are crystalline patents. If generic companies succeed in avoiding crystalline patents, they will be eligible to release late-stage drugs after the expiration of the substance patent in 2031. Although there is a long time left until the expiration of material patents, the reason why generic companies are interested in patent challenges is that K-Cab is performing very high in the prescription market. According to UBIST, a pharmaceutical market research firm, K-Cab surpassed 100 billion won in prescriptions in 2021, the third year of its launch. Last year, it booked a total of 92.2 billion won in the third quarter, surpassing 100 billion won for the second consecutive year. K-Cab is the flagship product of HK inno.N. It is a P-CAB-based gastroesophageal reflux disease treatment, and continues to grow with advantages such as that it is more effective than existing PPI-based products and can be taken regardless of before and after meals.
- Company
- Dupixent to be reviewed by DREC for reimb after 2 years
- by Eo, Yun-Ho Jan 12, 2023 04:29am
- The atopic dermatitis treatment ‘Dupixent’ has taken a step forward in extending reimbursement to children and adolescents. According to industry sources, the low-dose formulation (200mg) of Sanofi-Aventis Korea’s Dupixent (dupilumab) will be deliberated by the Health Insurance Review and Assessment Service’s Drug Reimbursement Evaluation Committee today on the 12th. This progress has been made 2 years after the company applied for the reimbursement extension in April 2021. It first took 7 months for expert opinion inquiries to begin on extending reimbursement of Dupixent to pediatric and adolescent patients, and the reimbursement standards for the indication were set in May last year. Being a high-priced new drug, the drug, which had difficulty being approved for reimbursement the first time, is having difficulty extending reimbursement as well. Although the specific indications may differ, the difference in speed of progress is evident when compared to other JAK inhibitors that applied for reimbursement extensions in atopic dermatitis, such as Lilly Korea’s ‘Olumiant (baricitinib),’ Abbvie Korea’s ‘RInvoq (upadacitinib)’ etc. The price of JAK inhibitors is also relatively lower than that of Dupixent, and the two drugs were both listed for reimbursement in May last year. Therefore, whether Dupixent will be able to pass DREC review and be extended reimbursement remains the focus of attention. However, the remaining journey will not be so easy for Dupixent. Being an RSA (Risk Sharing Agreement) drug as well as the addition of the separate 200mg dose, the drug will have to pass HIRA’s cost-effectiveness review process and complete drug pricing negotiations with the National Health Insurance Service. Meanwhile, the 300mg dose of Dupxient is currently reimbursed for adult patients aged 18 years or older with chronic severe atopic dermatitis who have had the condition for over 3 years and satisfy all three of the following criteria: ▲ who are unable to control their symptoms after receiving topical treatment for over 4 weeks, and ▲ unable to use systemic immunotherapies due to side effects or saw no response (50% or more decrease in EASI, EASI 50) after receiving treatment with systemic immunotherapies, and ▲ had an EASI score of 23 or higher before administering Dupixent.
- Company
- The effect of government regulation
- by Chon, Seung-Hyun Jan 11, 2023 05:59am
- More than 7,000 Rx drugs have withdrawn from the market over the past 3 years, the report showed. It is analyzed that this is due to the fact that the item license renewal system, which regularly checks the safety and efficacy of drugs, has been established and a series of market withdrawals due to continuous clinical re-evaluation. Some point out that the government's pressure to re-evaluate generic drug prices also encouraged the market to withdraw. According to the Ministry of Food and Drug Safety on the 11th, a total of 2,167 cases were withdrawn from the market last year, including the withdrawal and cancellation of Rx drugs. The number of Rx drugs permits was 93.8% higher than 1118. This means that there were about twice as many professional drugs in which market withdrawals entered the market as new markets last year. The number of withdrawals and cancellations of Rx drug licenses surged 68.1% from 1,600 in 2019 to 2,690 in 2020. In 2021, 2,595 Rx drugs were withdrawn, and more than 2,000 Rx drugs disappeared for the third consecutive year until last year. A total of 7452 Rx drugs have been withdrawn from the market over the past three years due to the withdrawal and cancellation of permits. A total of 3,796 Rx drugs returned permits over the three years from 2017 to 2019, with the number of products withdrawn from the market doubling over the next 3 years. It is analyzed that the number of products disappearing from the market has increased due to the combination of government regulatory movements such as drug item license renewal, clinical re-evaluation, and price of generic re-evaluation. It is pointed out that the withdrawal of expired drugs from the market has increased due to the establishment of the item license renewal system. The key to the drug item renewal system, which was based on the revision of the Pharmaceutical Affairs Act in 2012, is that drugs approved by health authorities must be re-certified every five years to maintain their licenses. Existing drugs on the market have been verified for safety and efficiency once every 16-20 years through a procedure called reevaluation. However, the renewal system was introduced because it was deemed necessary to operate a reasonable evaluation system according to rapid scientific development. Drugs licensed from January 1, 2013 are maintained only after submitting data related to safety and efficacy every five years and being judged suitable by the Ministry of Food and Drug Safety. If a drug whose item license expires does not submit data for renewal, the license will be canceled. An industry official explained, "After the implementation of the item license renewal system, even if there are no safety and efficiency problems, the practice of withdrawing products with low sales volume from the market has been established." The government's continuous revaluation policy is also cited as a factor that accelerates the product cleanup phenomenon. In the case of clinical re-evaluation, which rechecks efficacy and safety, it often leads to the withdrawal of the drug from the market. For example, in the case of Choline alfoscerate, a brain function improvement drug, more than half of the licensed products left with the start of clinical re-evaluation. In June 2020, the Ministry of Food and Drug Safety requested the submission of clinical trial data for Choline, and 57 pharmaceutical companies were approved for re-evaluation clinical plans. Initially, the Ministry of Food and Drug Safety ordered a total of 134 companies to re-evaluate Choline's clinical trials, but less than half of 57 companies were approved for re-evaluation clinical trial plans. This means that 77 companies gave up the re-evaluation of Choline and chose to withdraw from the market. Recently, some diagnose that the withdrawal from the Rx drug market has increased due to the re-evaluation of generic drug prices. In June 2020, the Ministry of Health and Welfare announced a plan to reevaluate the upper limit of drugs that maintain the previous drug price if Generic, which does not meet the highest price requirements, submits BA test and registered raw material drug data by February 2023. The re-evaluation of the generic drug price is a policy to apply the new drug price system, which took effect in July 2020, to the original generic. Each time one requirement is not met, the upper limit is lowered by 15%. Pharmaceutical companies should choose strategies to accept drug price cuts for consignment generics or avoid drug price cuts through additional investments. Requirements for the use of registered raw materials can be met through the replacement of raw materials and medicines. Pharmaceutical companies are in a situation where they have to choose between accepting drug price cuts or maintaining drug prices through conducting BA tests. Accordingly, pharmaceutical companies are actively conducting BA tests on licensed generics. The goal is to avoid lowering drug prices through permission to change the drug price by making generics through pharmaceutical research, conducting BA tests, and obtaining equivalent results. At this time, if the permission is changed while converting consignment manufacturing to its own manufacturing, the company is using a strategy that meets the requirements for 'BA test implementation'. For pharmaceutical companies, if additional investment is burdensome, they have no choice but to accept a reduction in drug prices. It is known that many generics often choose to withdraw from the market rather than accept drug price cuts. Some analysts say that reckless entry into generics, which did not take into account marketability before the government tightened regulations on generics, may have led to a withdrawal from the market after stricter regulations. In 2018, 175 items containing Valsartan, a hypertension treatment, were banned from selling due to the detection of excess impurities. At that time, the Ministry of Health and Welfare and the Ministry of Food and Drug Safety set up a "consultative body for improving the generic drug system" and began to come up with measures to curb the generics crisis. As the government hinted at the government's move to tighten regulations, pharmaceutical companies moved to install generic products in advance, temporarily increasing generic permits. The number of Rx permits reached 1,562 in 2018, which surged to 4,195 and 2,616 in 2019 and 2020, respectively. 374 of the Rx licensed in 2019 returned their permits. In other words, it withdrew from the market less than three years after it was approved by the government's move to tighten regulations. There are also more products that disappear from the market due to unexpected variables such as impurities. The Ministry of Food and Drug Safety decided in September 2019 to ban the sale of all products containing Ranitidine, citing excessive detection of NDMA, a carcinogenic substance.
- Company
- Rx Drugs permits have fallen by 73% in 3 years
- by Chon, Seung-Hyun Jan 10, 2023 05:35am
- Stair-type drug price systems and joint development regulations have dampened the entry power of generics Last year, the number of Rx drug permits decreased significantly. The number of market entries has been reduced by more than 70% compared to three years ago. Analysts say that the government's all-around regulatory pressure, such as the reorganization of the drug price system and regulations on joint development, has greatly reduced its entry into the generic market. According to the Ministry of Food and Drug Safety on the 9th, a total of 1,118 Rx drugs were approved last year. It decreased by 30.1% in a year from 1,600 in 2021. Last year, the number of Rx drug permits was less than half of the 2616 two years ago. Compared to 4,195 in 2019, it decreased by 73.3%. The number of Rx drug permits has decreased by 3077 compared to three years ago. The number of Rx drug permits averaged 130 per month to 1,562 in 2018, but more than doubled to 4,195 per month in 2019. In May 2019, 584 professional drugs were approved for a month. However, since 2020, the number of Rx drug permits has gradually decreased and seems to have regained the previous year's level. From October 2018 to July 2020, more than 100 Rx drugs were poured out every month, and in August 2020, Rx drug permits fell to less than 100 in 23 months. Last year, there were only four Rx drug licenses per month. It is analyzed that the number of generic permits, which account for the largest proportion of Rx drugs, has decreased. The reorganization of the drug price system is cited as a major factor in the decrease in the number of generic permits. The key to the revised drug price system, which took effect in July 2020, is to maintain the upper limit of 53.55% of the original drug before the expiration of the current patent only when generic products meet both the direct BA test and the use of the registered raw drugs. The reorganized drug price system includes a stepped drug price system in which the upper limit decreases as the salary registration period is delayed. If more than 20 generics are listed in the specific ingredient market, the upper limit of newly listed items will be up to 85% of the existing lowest price. Analysts say that the permission for the entire process of manufacturing consignment generics has decreased significantly due to the structure in which drug prices fall significantly if pharmaceutical companies do not develop generics themselves and conduct BA tests. Since more than 20 generics have already entered most markets with high marketability, late generics will inevitably lose their motivation to enter new markets as drug prices fall significantly due to the application of the step-type drug price system. Some say that regulations on the joint development of drugs, which took effect in July last year, have promoted the decline in generic permits. The revised Pharmaceutical Affairs Act, passed at the plenary session of the National Assembly in May last year, calls for limiting the number of improved new drugs and generics that can be approved as a clinical trials. If all manufacturing processes are manufactured the same way with the same prescription and manufacturing method at the same manufacturing plant as the pharmaceutical company's drug that directly conducted the BA test, the use of BA data is limited to three times. This means that only four generics can be approved for one BA test. Clinical trial data can also be agreed to only three items other than drugs from direct-performing pharmaceutical companies. In the past, dozens of pharmaceutical companies often received consignment generic licenses with the same data if certain pharmaceutical companies were approved for generics through BA tests, but joint development regulations have made it impossible to "unlimited generic replication." Critics point out that the government provided the cause of the surge in Rx permits in 2019 and 2020. The government's move to tighten regulations on generics has led to a surge in generics permits. In 2018, 175 items containing valsartan, a hypertension treatment, were banned from selling due to the detection of excess impurities. At that time, the Ministry of Health and Welfare and the Ministry of Food and Drug Safety set up a "consultative body for improving the generic drug system" and began to come up with measures to curb the generic crisis. As the government's move to tighten regulations showed, pharmaceutical companies moved to install generic products in advance, temporarily increasing generic permits. The number of generic permits surged due to the government's move to tighten regulations and returned to the previous level after the system was reorganized.