- LOGIN
- MemberShip
- 2025-12-24 02:10:27
- Company
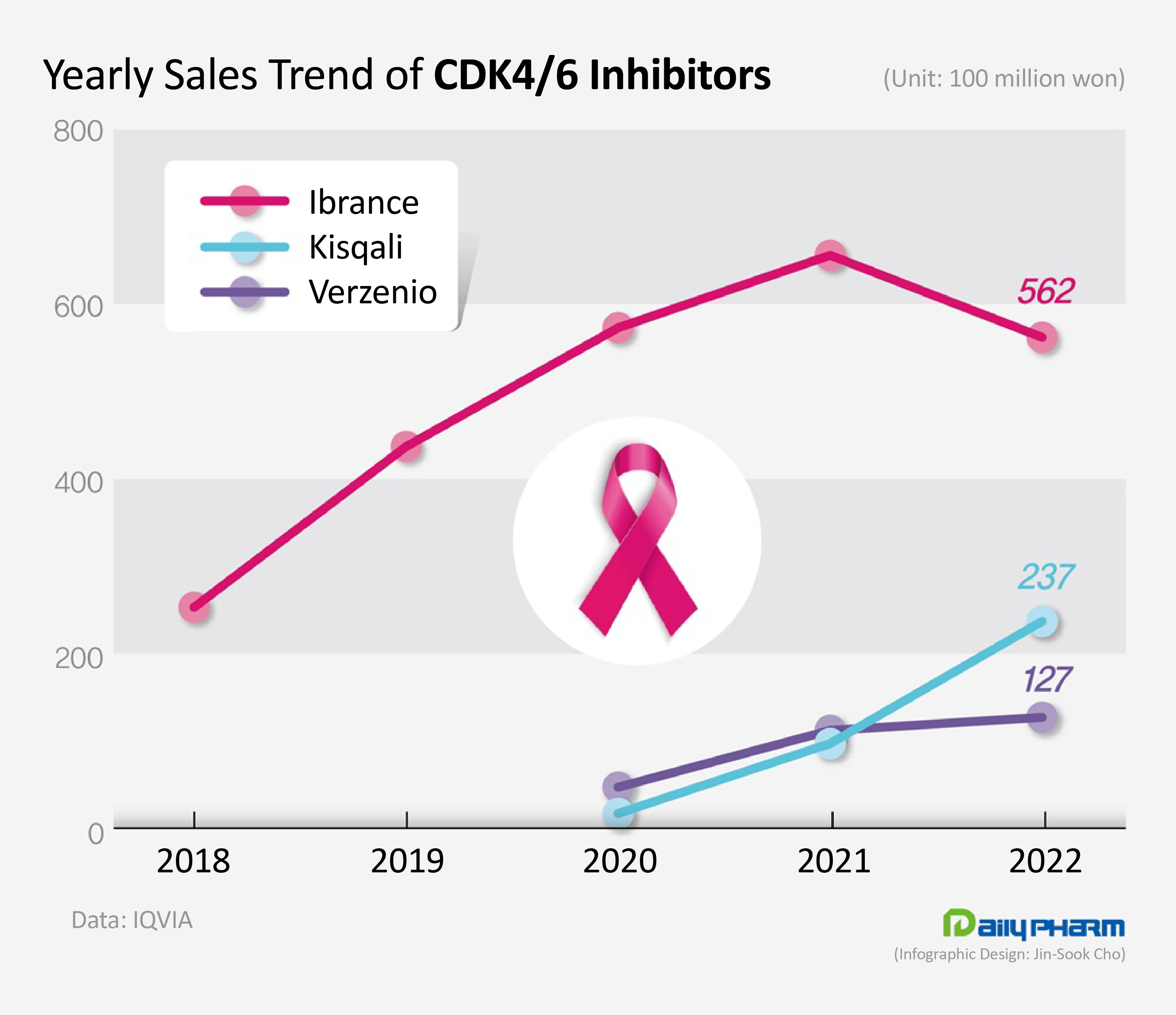
- Sales of Kisqali surge, Ibrance decline
- by Jung, Sae-Im Mar 09, 2023 06:00am
- The market for cyclin-dependent kinases (CDK) 4/6 inhibitors that are used to treat metastatic breast cancer have undergone drastic changes last year. Sales of Ibrance, which used to dominate the market, had faltered, while the latecomer Kisqali rapidly expanded its share in the market . According to the market research institution IQVIA on the 8th, the domestic market for CDK 4/6 inhibitors increased 7.1% YoY from KRW 86.5 billion in the previous year to ₩92.6 billion last year. CDK, cyclin-dependent kinases, control cell division and growth. CDK 4/6 inhibitors selectively inhibit CDK 4/6 to suppress the proliferation of cancer cells. These drugs are mainly used to treat hormone receptor (HR)-positive or human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer, which accounts for 60% of all breast cancers. Since the introduction of the first-in-class CDK 4/6 inhibitor, Pfizer’s Ibrance (palbociclib), Novartis’s Kisqali (ribociclib), and Lilly’s Verzenio (abemaciclib) are also available in the market. Ibrance, which overtook the market as the 'first-in-class' drug, experienced its first decline in sales last year. Ibrance’s sales fell 14.3% YoY from the KRW 65.6 billion in the previous year to record sales of KRW 56.2 billion last year. The drug, which had shown repeated growth since its launch, faced a decline in sales for the first time in six years. The first CDK 4/6 inhibitor Ibrance was developed by Pfizer and released in Q4 2016 in Korea. The drug offered a new treatment option for patients with HR+/HER2- metastatic breast cancer that were left to use chemotherapy for their disease that cannot be controlled with hormone therapies like aromatase inhibitors. Upon its release, Ibrance was received with high expectations in the market, being the first drug to demonstrate a PFS of over 2 years. Ibrance’s sales rose sharply from KRW 6.6. billion in 2017 to KRW 25.3 billion in 2018, KRW 43.7 billion in 2019, and then KRW 57.3 billion in 2020. The sales decline started with the introduction of its latecomers. Kisqali and Verzenio entered the market in 2020 and started expanding their presence ever since. Among the latecomers, Kisqali has been showing marked growth. Kisqali’s sales rose 143.6% YoY from the KRW 9.7 billion earned in the previous year to record KRW 23.7 billion last year. Although it had the lowest share of the market with annual sales of less than KRW 10 billion until 2021, its sales had risen to the KRW 20 billion range last year and exceeded Verzenio’s sales. The fact Kisqali is the only treatment option among CDK4/6 inhibitors that can be used in premenopausal breast cancer patients seems to have contributed to its rapid growth. Ibrance and Verzenio are only indicated for the treatment of postmenopausal women with metastatic breast cancer. However, Kisqali demonstrated its effect in premenopausal patients in clinical trials. Unlike in the West where premenopausal patients account for less than 30% of the patient population, these women account for 55% of the patient population in Korea, which explains the increased use of Kisqali. However, sales of Verzenio, which have risen sharply in 2021, had also slowed down somewhat. Verzenio’s sales increased 13.7% YoY from the KRW 11.2 billion in the previous year to KRW 12.7 billion last year. Verzenio is seeking to expand its market base this year after it had become the only CDK4/6 inhibitor allowed for use in early breast cancer. The Ministry of Food and Drug Safety additionally approved Vezenio as an adjuvant treatment for patients with HR+ /HER2- type lymph node-positive, early breast cancer at high risk of recurrence. This is the first time a new drug was approved for early breast cancer after the approval of aromatase inhibitors.
- Company
- Childhood dementia Tx Xenpozyme to soon land in KOR
- by Eo, Yun-Ho Mar 09, 2023 06:00am
- The first childhood dementia treatment is expected to be commercialized in Korea soon. According to industry sources, the Ministry of Food and Drug Safety is conducting the final review to approve Sanofi Genzyme’s treatment for acid sphingomyelinase deficiency (ASMD)m ‘Xenpozyme (olipudase alfa).’ Starting with Japan in March, the drug was also approved in Europe in July and by the US FDA in August and received Breakthrough Therapy designation in the countries. The drug received final review in July and September in Europe and the US, respectively. The efficacy of Xenpozyme, the only existing ASMD treatment, was identified through the ASCEND and ASCEND-Peds trials. The ASCEND trial evaluated the efficacy and safety of Xenpozyme in 36 adult patients with ASMD type A/B or type B. The patients were randomized to receive Xenpozyme or a placebo for 52 weeks (primary analysis). At Week 52, Xenpozyme improved pulmonary function from baseline by 22% in the predicted diffusing capacity of carbon monoxide (DLco). Compared with the 3% improvement shown in the placebo group, the difference between the two treatment arms of 19% was statistically significant. Also, at Week 52, patients treated with Xenpozyme had a mean reduction in spleen volume by 39.5% compared with the 0.5% increase in the placebo group. All patients that were treated with Xenpozyme showed an improvement in one or two primary endpoints. The single-arm ASCEND-Peds trial studied 20 pediatric patients younger than 12 years of age with ASMD type A/B or type B. The primary objective of the trial was to evaluate the safety and tolerability of Xenpozyme for 64 weeks, and the explored efficacy endpoints of progressive lung disease, spleen, and liver enlargement, and platelet count were also explored in the trial. The nine patients who could take the test for DLco in the trial showed a 33% improvement in diffusing capacity after 1 year. The patients also showed a mean reduction in spleen volume of 49%. Meanwhile, ASMD is caused by the lack of an enzyme needed to break down a complex lipid, called sphingomyelin, which accumulates in the liver, spleen, lung, and brain. Patients with ASMD experience enlarged abdomens at 3 to 6 months of birth. The most severely affected patients have profound neurologic symptoms and rarely survive beyond two to three years of age.
- Company
- Hemlibra is also effective for mild and secondary hemophilia
- by Kim, Jin-Gu Mar 09, 2023 06:00am
- JW Pharma announced on the 6th that Phase III clinical trials that proved the effects and safety of patients with type hemophilia have been published in the online edition of the Lancet Hematology 2023, an international journal. Hemlibra is a type A hemophilia disease caused by the deficiency of factor XIII. It is the only anti-antibody patient and non-antibody patient with resistance to the existing therapeutic agent (8-factor formulations), and the prevention effect persists with subcutaneous injections for up to 4 weeks. Type A hemophilia is divided into mildness (more than 5% to less than 5% to less than 40%), moderate (1% or more to 5% or less), and severe (less than 1%) according to the figures of factor XIII activation. Hemlibra has been licensed in Korea for severe A-type hemophilia prevention therapy. 18 professors, including Claude Bernard Lyon 1 University, conducted about 55 weeks of clinical trials for 72 patients with mild and secondary A-type hemophilia in 22 institutions, including Europe, North America, and South Africa. The researchers administered to patients once a week for the first four weeks of Hemlibra, and then selected once a week or once ▲ 4 weeks once a week to evaluate the bleeding volume and thrombosis adverse events. Annual Bleed Rate, which was 10.1 times, decreased by 0.9 times after hembra administration. Among them, joint bleeding and natural bleeding ABRs, which required treatment, were 0.2 times. 21 mild patients have decreased from 20.2 times before Hemlibra administration, and 2.4 times after administration, while moderate patients decreased from 6.0 to 2.2 times. The ABR of the patient group, which had been treated with the existing coagulation factor treatment as a preventive therapy, was improved from 8.0 to 2.4 times after clinical 12.2 times before clinical trials and bleeding groups. Eight patients were not bleeding during the clinic. In terms of safety, 15 patients had minor side effects related to the injection site, but no death or thrombectomy microvascular disease occurred. Based on these clinical results, Hemlibra was approved by the European Union (EU) as a preventive treatment for patients with non-antibody aid type hemophilia. An official of JW Pharma said, "This clinical trial indicates that Hemlibra is effective in preventing bleeding for patients with mild and secondary A-type hemophilia." said. Hemlibra was developed by Japan's Chugai Pharmaceutical, a subsidiary of Global Pharm Roche. JW Sino -Pharmaceuticals secured domestic development and copyrights of Hemlibra in 2017 and launched it as a treatment for severe A-type hemophilia in 2020.
- Company
- Becton Dickinson achieves a 90% customer satisfaction rate
- by Eo, Yun-Ho Mar 08, 2023 05:53am
- Becton Dickinson (BD Korea), a multinational medical device and equipment manufacturing company, achieved a customer satisfaction rate of 90%. The company conducted a customer satisfaction survey for domestic pharmaceutical and bio customers and announced the results on the 6th. The survey was conducted between January 9 and February 3 of this year for a total of 74 domestic pharmaceutical and bio customers that supply drug delivery systems including pre-filled syringes from the BD Pharmaceutical Division. It was conducted online for about a month until the day. The survey consisted of a total of 25 questions in five areas: product ordering and delivery, quality and technical support, understanding customer needs, prospects for partnerships, and online resources. As a result of the survey, more than 90% of customers responded that they were satisfied with BD Korea's order, delivery, and supply system. Out of BD Korea's services, items of interest were answered in the order of ▲approval support (31.52%) ▲container tightness test (25.0%) ▲device, and drug convergence product test (22.83%). BD Korea provides customized permit support services optimized for each country's regulations, requirements, and guidelines in various countries including Korea, Europe, the United States, China, and Malaysia. Representatively, there are RA services (Regulatory Support Service), which supports convergence product registration, and Your Path (BD YourPathTM), which provides product and test permit consulting online and offline. BD Korea merged with ZebraSci Inc., a company specializing in convergence product testing, in 2021 to provide various service items from the initial development stage of customers' products to clinical and commercialization. Through this, BD Korea has expanded its capabilities to smoothly support customers' goals and timely market entry during the development of convergence products. In addition, in a survey of innovative drug delivery system products and service solutions, ▲ technology that enables product traceability (30.82%) ▲ wearable devices for injection (29.56%) ▲ reusable autoinjectors (18.87%) were the main areas of interest for customers. appear. In addition, as a result of a satisfaction survey on BD Korea's technical support, data provision, and quality policy, it was tallied that more than 80% were satisfied. BD Korea plans to hold a professional consulting meeting with a BD Global license support manager for pre-booked customers at the International Pharmaceutical, Bio, and Cosmetics Technology Exhibition (COPHEX), which will be held from April 18 to 21 this year.
- Company
- Hemophilia A treatment market sales were 66 billion won
- by Kim, Jin-Gu Mar 08, 2023 05:53am
- Hemlibra Last year, sales of hemophilia A treatment in hospitals and clinics were tallied at 66 billion won. While existing major drugs are sluggish in hospitals and clinics, JW Pharmaceutical's Hemlibra, which recently joined the market, has risen in sales for two consecutive years, rising to No. 2 in the market. In the pharmaceutical industry, sales of Hemlibra are expected to increase further as benefits for Hemlibra are expected to increase in the first half. As a result, there is a prospect that the hemophilia A treatment market, which has been led by GC Pharma, will be greatly shaken. According to IQVIA, a pharmaceutical market research institute on the 7th, sales of hemophilia A treatment to hospitals and clinics last year were 66.2 billion won. It decreased by 15% compared to 78.2 billion won in 2021. Sales data generated by medical institutions operated by the Hemophilia Foundation were excluded from the tally. In the pharmaceutical industry, if this data is included, it is estimated that the total sales volume would have been maintained at the level of the previous year. While the overall market size of hospitals and clinics has been reduced, JW Pharmaceutical Hemlibra has succeeded in increasing sales for two consecutive years. Last year, Hemlibra sales were 7.6 billion won, up 5% from 7.2 billion won in 2021. Although the increase in sales last year was not large, it is analyzed that it rose to the second place in the market at once as major competing products performed poorly overall. Hemlibra is a hemophilia A treatment introduced by JW Pharmaceutical in Korea. In 2017, JW Pharmaceutical secured exclusive domestic development and sales rights for Hemlibra from Chugai Pharmaceuticals. In January 2019, domestic item permission was obtained, and in May 2020, it was listed as a salary and released in earnest. In the first year of its release, it recorded sales of 2.1 billion won. It is evaluated that Hemlibra succeeded in making a soft landing in the market by improving the convenience of administration. Hemlibra is the first subcutaneous injection formulation among hemophilia A treatments. Existing treatments required patients to find a vein and inject themselves. In particular, since many of the patients are children and adolescents, there was not a little inconvenience with the intravenous injection formulation. Existing major products such as Takeda Pharmaceutical's Adynovate, Novo Nordisk's Novoseven, and GC Pharma's GreenMono saw a simultaneous decrease in sales. Adynovate's sales last year were 19.5 billion won. It decreased by 15% compared to 22.9 billion won in 2021. Adynovate peaked at 27.7 billion won in 2019, and sales declined for three consecutive years until last year. However, it still ranks first in the market. In the case of Novoseven, it decreased by less than half from 14 billion won in 2021 to 5.5 billion won last year. The market ranking fell from 2nd to 5th. It recorded sales of 19.5 billion won in 2018, but since then, it has steadily decreased, and the new sales volume has reached a quarter of the level in four years. Green Mono decreased from 8.3 billion won in 2021 to 6.6 billion won last year. It's ranking in the hemophilia A treatment market fell from third to fourth. The pharmaceutical industry predicts that Hemlibra will grow more rapidly in the future. This is because the scope of benefits is expected to expand in the first half of this year. Last February, the HIRA recognized the adequacy of Hemlibra's reimbursement as a 'prophylactic treatment for patients with severe non-antibody hemophilia A'. JW Pharmaceutical plans to begin negotiating the drug price of Hemlibra with the NHIS soon. It is expected that the benefit increase will be decided within the first half of this year at the earliest. Currently, Hemlibra is covered only by antibody patients resistant to existing treatments. It is known that 9 out of 10 hemophilia A patients in Korea do not have antibodies. According to the 2019 Hemophilia White Paper, there are 1,746 patients with hemophilia A in Korea, of which 1,589 are non-antibody patients, accounting for 91%. In effect, Hemlibra's salary range is expanded 10 times, and industry insiders predict that related sales will surge in the process. Attention is focused on whether JW Pharmaceutical will emerge as a strong competitor to GC Pharma in the hemophilia A treatment market due to Hemlibra's coverage expansion. GC Pharma dominates the domestic hemophilia A treatment market. GC Pharma sells its own products, GreenMono and Greengene F. In addition, Takeda Adynovate and Adynovate, the No. 1 and No. 3 products in the market, will be jointly sold. The combined sales of the four products last year were 35.6 billion won, accounting for more than half of the hemophilia A treatment market last year.
- Company
- 'Vabysmo’s 4mth dosing interval may reduce patient burden'
- by Jung, Sae-Im Mar 08, 2023 05:53am
- A new drug for Neovascular (Wet) Age-Related Macular Degeneration (nAMD) that offers treatment effect with once every 4-month administration has landed in Korea. The new option is expected to greatly improve the convenience of administration in domestic patients whose number has been increasing with Korea’s aging population. Roche Korea held a press conference on the 7th to celebrate the approval of Vabysmo (faricimab) at its headquarters in Seocho-gu, Seoul. Vabysmo is the first bispecific antibody that targets both the VEGF that is commonly targeted by existing ocular disease treatments as well as angiopoietin-2 (Ang-2) that are considered to be the cause of retinal disease to block both pathways. MOA of Vabysmo Vabysmo has demonstrated non-inferiority over Eylea in improving vision outcomes in a Phase III study and was approved in Korea in January as a treatment for the treatment of patients with Neovascular (Wet) Age-Related Macular Degeneration (nAMD) and Diabetic Macular Edema (DME). With the approval, the total number of AMD treatments available in Korea increased to 4 – Vabysmo (Roche), Eylea (Bayer), Lucentis (Novartis), and Beovu (Novartis). nAMD is considered one of the 3 major causes of blindness in the elderly aged 65 or older. The importance of its treatment is increasing with the rising number of patients in the rapidly aging society. During his presentation, ophthalmologist Kim Jae-hui, (Kim’s Eye Hospital) said, “The widely used nAMD treatments Eylea and Lucentis are effective but have their limitations. One is that the drugs have to be injected frequently for a longer period of time to maintain the patients' vision and anatomical improvements. Also, the response may be limited even with treatment depending on the patient. In this sense, expectations are rising on treatment effect as the only drug that also inhibits Ang-2. “ Ophthalmologist Jae-Hui Kim (Kim The bispecific antibody Vabysmo has demonstrated that it can produce comparable effects to existing treatments while significantly increasing the dosing interval. After the first 4 doses are administered by intravenous injection every 4 weeks, the dosing interval for patients with no disease activity can be extended to every 16 weeks (4 months). In other words, the effect that existing treatments offered with every 1-2 month administration can be achieved with up to every 4-month administration of Vabysmo. In two Phase III trials conducted on 1,329 patients, patients who received Vabysmo at dosing intervals of up to 4 months showed non-inferiority over its comparator, the 2-month interval treatment Eylea, in improving vision outcomes after 1 year of treatment. Over half of the Vabysmo-administred patients were maintaining the 4-month dosing interval after 1 year of treatment. Kim expected that the introduction of Vabysmo and its longer dosing interval would benefit patients who had felt burdened by the frequent injections to the eye. Kim said, “In many cases, it was difficult to reproduce the results that were found in the clinical setting on-site because patients cannot receive regular injections in the long term as strictly as they would have had in clinical trials. So, their effect was reduced in line with the reduced number of injections the patients received. In this sense, I have higher hopes for Vabysmo as it only needs to be injected once every four months, which reduces patient burden. During practice, I often encountered patients whose edema does not go away with existing treatments, but data has shown that Vabysmo can remove edema at a higher rate. I think this will benefit the patients who have had shown limited response to existing treatments” At the press conference, Roche Korea expressed the company's plans to promptly supply Vabysmo in Korea through quick reimbursement listing. In-Hwa Choi, Lead at Roche Korea, said, “Vabysmo has been approved in 50 countries worldwide and is reimbursed in A7 countries and Australia. We have already applied for its reimbursement at the end of last year before it was approved in Korea.”
- Company
- Self-administered Kynteles lands at general hospitals
- by Eo, Yun-Ho Mar 08, 2023 05:52am
- The self-injectable formulation of the inflammatory bowel disease treatment ‘Kynteles’ can now be prescribed at general hospitals in Korea. According to industry sources, the subcutaneous injection formulation of Takeda Pharmaceuticals Korea’s Kynteles (vedolizumab) has passed the drug committee (DC) reviews in tertiary hospitals such as Samsung Medical Center and Seoul Asan Medical Center and other general hospitals including Kyungpook National University Hospital, Yeungnam University Medical Center, and Inje University Haeundae Paik Hospital. The company has been quickly expanding its prescription area after the drug was listed for reimbursement in December last year. Kynteles is reimbursed in Korea if the patient ▲writes a ‘patient administration journal’ that is managed by his/her healthcare institution within 14 weeks of the first administration. For long-term prescriptions, ▲up to 2 weeks’ worth of prescriptions is allowed per prescription upon discharge, and up to 4 weeks’ worth of prescriptions is allowed for outpatient care patients. Also, up to 8-12 weeks’ worth of administration is approved for reimbursement in patients who show stable disease activity after 24 weeks of administration with no side effects. The subcutaneous injection formulation of Kynteles, ‘Kynteles Prefilled Pen inj.’ can be self-administered by the patient without visiting hospitals, and was approved for the same efficacy and effect as ‘Kynteles Inj,’ the intravenous injection formulation, in Korea on February 17. In addition to the existing strength held by the intravenous injection formulation of having a short administration period of 30 minutes, the added reimbursement of the subcutaneous injection formulation has provided a wider treatment option for Korean patients. The subcutaneous formulation of Kynteles showed a similar rate of clinical remission to that of the intravenous injection in the VISIBLE trials. In the VISIBLE 1 study that was conducted on adult patients with ulcerative colitis, the subcutaneous formulation showed comparable improved improvement with the intravenous formulation in terms of efficacy, safety profile, and tolerability. The VISIBLE 2 trial that evaluated the subcutaneous formulation as a maintenance treatment in adults with Crohn’s disease also confirmed the significant improvement in results, demonstrating the new formulation’s effect as a maintenance treatment. Kynteles is a biological agent used to treat patients with moderate-to-severe active ulcerative colitis or Crohn's disease. As the only anti-integrin therapy among inflammatory bowel disease treatments, the drug owns a safety profile that does not cause systemic immunosuppressive activity.
- Company
- Celltrion Healthcare published clinical result of Remsima SC
- by Hwang, Jin-joon Mar 08, 2023 05:51am
- An official from Celltrion Healthcare is giving a presentation on Remsima SC at ECCO. (Photo by Celltrion Healthcare)Celltrion Healthcare announced on the 6th in Copenhagen, Denmark, that it was holding the '2023 European Crohn's Disease and Colitis Society (ECCO)' for four days from the 1st (local time), and 'Remsima SC' was held for the purpose of US approval. announced that it had unveiled a new global clinical trial of '. The first clinical trial is the result of analyzing the efficacy and safety of Remsima SC compared to placebo during maintenance therapy after administering Remsima to patients with Crohn's disease (CD). It was released as a digital oral presentation online. 343 patients with moderate to severe CD were randomly assigned to the Remsima SC treatment group and the placebo control group in a 2:1 ratio at week 10 and compared at week 54. Clinical outcome The primary endpoint, clinical remission (CR), was 62.3% for Remsima SC and 32.1% for placebo. In the endoscopic response (ER), Remsima SC 51.1% and placebo 17.9%, a statistically higher efficacy result than the control group was confirmed. No new safety issues were identified with Remsima SC maintenance treatment. The second clinical trial was released through a poster presentation as a result of analyzing whether Remsima SC maintained a statistically significant advantage over placebo in phase 3 clinical trials for patients with ulcerative colitis (UC). After 438 patients with UC were treated with Remsima up to week 10, they were randomly assigned to receive Remsima SC or placebo in a 2:1 ratio, and data from week 54 were compared. The CR set as the primary evaluation index for clinical results was 43.2% in the Remsima SC-administered group, higher than 20.8% in the placebo control group. No new safety issues were found in the clinical trial. The results of the post-clinical phase 1 post-analysis confirming the correlation between high serum trough concentration and low immunogenicity of Remsima SC were also released as a poster. Predictors of Immunogenicity in IBD Patients Treated with Infliximab: According to CT-P13 SC Phase 1 Post-Clinical Analysis', maintenance treatment with Remsima SC confirmed that the proportion of patients whose blood concentration reached a certain threshold or higher was higher than that of patients receiving an intravenous injection. done. Through this, indices such as antibody to the drug (ADA) and neutralizing antibody (NAb) involved in the immune process were lower, confirming the potential advantage of Remsima SC in terms of immunogenicity. Celltrion Healthcare also published three posters, including 'Network meta-analysis for comparative evaluation of the efficacy of Infliximab IV and SC and Vedolizumab IV and SC in the maintenance treatment of patients with Crohn's disease and ulcerative colitis'. presented at the conference.
- Company
- Exkivity applies for reimbursement
- by Eo, Yun-Ho Mar 07, 2023 05:38am
- Exkivity, an anti-cancer drug targeting EGFR exon 20 insertion mutation, is aiming for insurance coverage. Takeda Korea recently submitted a reimbursement application for Exkivity, a treatment for non-small cell lung cancer (NSCLC) with an EGFR exon 20 insertion mutation. This drug targets the same biomarker as Janssen's Rybrevant but differs in that it is an oral drug. EGFR Exon 20 insertion mutation is a new biomarker that has recently attracted attention in the field of non-small cell lung cancer. Currently available anticancer drugs are suitable for Exon19 deletion or Exon21 L858R substitution mutation, which are commonly found in EGFR mutations, but EGFR Exon20 was still a blind spot. Accordingly, it remains to be seen whether GFR exon 20 insertion mutation non-small cell lung cancer targeting anticancer drugs can be listed in Korea. In the case of Rybrevant, it failed to cross the HIRA barrier after applying for benefits once. Rybrevant proved its efficacy through a phase 1/2 study conducted on 114 patients with non-small cell lung cancer with an EGFR exon 20 insertion mutation who had previously received platinum-based chemotherapy. Clinical results, in the patient group who took Rybrevant 160 mg, the ORR evaluated by IRC was 28% and the mDOR was 17.5 months. In particular, the median reaction time after administration of Rybrevant was 1.9 months, confirming that the drug's effect appears quickly from the beginning of treatment. mPFS was 7.3 months and mOS was 24.0 months. The safety profile was also found to be favorable. The most common adverse reactions were diarrhea, rash, and fatigue, which can be managed by adjusting the dose.
- Company
- Boryung officially launches Zepzelca
- by Kim, Jin-Gu Mar 06, 2023 05:56am
- Boryung announced on the 28th that it has officially launched Zepzelca, a new small-cell lung cancer drug, in Korea. Zepzelca is a new anticancer drug developed by PharmaMar S.A. It is used for metastatic small-cell lung cancer that has failed first-line platinum-based chemotherapy. Zepzelca is a new drug with a mechanism that simultaneously shows 'apoptosis of cancer cells through inhibition of DNA transcription' and inhibition of cancer cell proliferation, immune checkpoint action, and angiogenesis through suppression of transcriptional activity in Tumor-Associated Macrophage (TAM). Zepzelca was approved by the Ministry of Food and Drug Safety in September of last year and will be distributed to medical institutions in earnest through this official launch. It was released in July 2020 in the US. Zepzelca has established itself as a representative second-line treatment for small-cell lung cancer in the United States, with sales of $535 million until last year. Currently, more than 40% of patients with small cell lung cancer are prescribed Zepzelca as a second-line treatment. In Korea, Boryung has held exclusive sales and distribution rights for Zepzelca since 2017. Boryung expects the use of Zepzelca to expand as there are not many types of second-line or higher small-cell lung cancer treatments in Korea. According to the literature supporting the approval of Zepzelca published in The Lancet Oncology, an overall response of 35% based on the entire patient group, the average duration of response of 5.3 months, ease of administration given once every 3 weeks, and manageable side effects such as clinical benefits such as existing drug The contrast effect is evaluated as excellent. For this reason, Zepzelca is also recommended in the NCCN and ESMO guidelines. Young-seok Kim, head of Boryung Onco Division, said, "So far, the options for second-line treatment have been limited for patients with small cell lung cancer who have failed platinum-based chemotherapy."