- LOGIN
- MemberShip
- 2025-12-23 19:18:55
- Company
- “Need to increase support for new AI drugs in Korea”
- by Jung, Sae-Im May 22, 2023 05:42am
- “Although artificial intelligence (AI) new drug development ecosystem is being created in Korea, there are still many areas that we are lacking at a global level in terms of manpower or investment scale. Chinese companies that were established around the same time received more than KRW 500 billion in investments, while Korean companies only received KRW 87.8 billion, which delayed their growth. It's time to make efforts to expand this ecosystem while building success stories through step-by-step collaboration.” Wooyeon Kim, Head of Korea Pharmaceutical and Bio-Pharma Manufacturers Association (KPBMA)’s AI New Drug Development Support Center stressed so at the ‘Pharmaceutical-Bio AI Innovation Forum’ that was held at Lotte Hotel Seoul in Sogong-dong on the 19th. Wooyeon Kim, Head of the AI New Drug Development Support Center At the event, which was hosted by KPBMA, Kim explained that Korea’s AI drug development market has grown steadily over the past 5 years, forming a virtuous cycle ecosystem. Companies and research centers are actively collaborating with AI new drug development companies. There were 88 cases of such collaborative efforts being made for drug repositioning, target discovery, and candidate substance discovery including partnerships between ▲ Boryung Pharmaceutical - Oncocross ▲ Samsung Seoul Medical Center - NetTargets ▲ Dong-A ST - Pharos iBio, etc. The government has also been continuing its efforts. Ministry of Science and ICT has been investing a total of KRW 18 billion for the discovery of innovative new drugs using AI for 5 years since 2022. The Ministry of Health and Welfare is also planning to build a Korean-style Rosetta Fold (an AI-applied protein structure prediction and analysis platform) next year and promote technology matching between pharmaceutical companies and AI companies. In addition, supports for projects such as medical big data construction and AI-based new drug development education are also being carried out. However, Korean AI drug development companies are still experiencing difficulties in securing talent and attracting investment. The stark difference stands out when compared to the global environment. Kim cited the cases of Standigm, a Korean company, and XtalPi, a Chinese company. Although the two companies were founded at a similar foundation time and owned similar levels of technology. However, Standigm had received KRW 87.8 billion in investments while XtalPi attracted 6 times as much, which was KRW 533.8 billion. XtalPi has 700 professionals, which is 10 times more than the 54 experts at Standigm. On this, Kim said, “Although the two companies were established at a similar period, XtalPi has achieved far much on the global stage due to its overwhelming investment and manpower over Standigm. In this graph that shows the growth rate of each company, XtalPi has shown faster growth.” In order to revitalize the ecosystem, Kim saw the need to accumulate successful collaboration cases in each stage of development, and encouraged research on matching supply and demand in the area. "There should be more cases that show companies that drug development can be accelerated and efficiency increased through the use of AI in the industry." Kim added, "This difference is more due to the difference in investment and manpower rather than technology. Although the AI drug development ecosystem in Korea has grown considerably, we are still lacking in many, many areas.” Kim also asked for the government’s closer attention in the field as global competition is taking place. He said, "A lot of investment is being made, mainly in the US and China, for the development of new AI drugs. Korea should also make bold investments and accumulate results step by step." Kim stressed that Korea should also strive to nurture convergent talents that understand AI. Kim said, "Demand for talent training is very high. More than 3,800 people attended the 385 hours of lectures that were conducted at our center. In particular, as convergence is very important in this field, we need to establish a system that continues to nurture convergent talent."
- Company
- Attempts to develop new drugs for hypertension
- by Hwang, Jin-joon May 22, 2023 05:41am
- Opinions were raised that it would be difficult for candidates under development as new drugs for hypertension, such as Baxdrostat, Aprocitentan, and Firibastat, to replace existing drugs. It is expected that it will fill the unmet demand rather than take the place of the prescribed treatment. Choi Ung-gil, professor at Konkuk University Chungju Hospital, is giving a presentation. (photo by Dailypharm) Professor Woong-Gil Choi of Chungbuk National University Hospital held a hypertension drug treatment update session at the '2023 Korean Society of Hypertension Spring Conference' held at COEX in Daegu on the 20th and said, "Major hypertension drug candidates are existing drugs rather than replacing drugs already prescribed in clinical settings. It will be a drug that can help the unfilled part.” The reason why the development of a new antihypertensive drug is needed is that it is a method to treat treatment-resistant hypertension. Professor Woong-Gil Choi explained, "Although the treatment control rate of hypertension has improved a lot, there is no further development after exceeding 70%." According to Professor Choi, major antihypertensive drug candidates include Baxdrostat, Aprocitentan, and Firibastat. Baxdrostat is a candidate substance secured by AstraZeneca, a global pharmaceutical company when it acquired CinCor Pharma, a US bio company. It is a new drug candidate for hypertension in the class of aldosterone synthase inhibitors (ASI). The efficacy of Baxdrostat in lowering blood pressure was confirmed in phase 2 clinical trial (BrigHTN) conducted on patients with treatment-resistant hypertension. In phase 2 clinical trial (HALO) conducted for uncontrolled hypertension patients taking up to two blood pressure medications, statistical significance was not achieved in the primary endpoint, but systolic blood pressure was reduced in subgroup analysis. Phase 3 clinical trials are expected to begin at the end of this year. Professor Choi said, “Baxdrostat appears to be relatively beneficial for hypertensive hypertension,” and “it is expected to give benefits to patients with primary aldosterone and metabolic syndrome.” Aprocitentan is a new drug candidate for hypertension being jointly developed by global pharmaceutical company Janssen and Swiss bio company Idorsia. It is an endothelin receptor antagonist. Applications for product approval were submitted to the FDA and EMA in December of last year and January of this year, respectively. Clinical data of Baxdrostat (Photo by Dailypharm) Aprocitentan has been confirmed to have a significant blood pressure-lowering effect in patients whose hypertension is not well controlled despite taking three or more existing treatments in phase 3 clinical trials (PRECISION). Professor Choi explained, "Although Aprocitentan has a stronger blood pressure lowering effect when used with other drugs, care should be taken about the fact that edema occurred after using the drug compared to placebo." Firibastat is a candidate material being developed by Quantum Genomics, a French biotech company. Firibastat is a candidate in the class of brain aminopeptidase A inhibitors. It is a mechanism that suppresses the production of angiotensin 3 in the brain's renin-angiotensin system (Brain RAS). Firibastat's efficacy with statistical significance was confirmed until the phase 2 clinical trial was conducted for patients with treatment-resistant hypertension. Afterward, it failed to achieve the primary evaluation index in phase 3 clinical trial (FRESH). Quantum Genomics is revising its development strategy to find new indications after the early termination of clinical trials. Professor Choi predicted, “There are still many cases of treatment-resistant hypertension, but if the development of a new drug for hypertension is successful, it will be possible to increase the treatment effect by adding it to existing drugs.”
- Company
- Forxiga price cut enforcement suspension extended
- by Jung, Sae-Im May 19, 2023 05:48am
- A decision on whether to suspend the execution of drug price cuts for AstraZeneca Korea’s diabetes treatments Forxiga and Xigduo is expected to be decided at the end of this month at the earliest. The temporary suspension period, originally until May 19, is also extended. On the 16th, the first division of the Seoul Administrative Court held an interrogation date for the suspension of drug price cuts filed by AstraZeneca Korea against the Ministry of Health and Welfare. The court, which conducted a private interrogation, decided to decide whether to quote a suspension of execution between the end of this month and the beginning of next month. As the court’s decision on whether to suspend enforcement is over 19 days away, the temporary suspension period for drug price cuts is also expected to be extended. Previously, the court had temporarily maintained the drug price until May 19, the scheduled date of the suspension trial. Forxiga and Xigduo, SGLT-2 inhibitors, are blockbuster products that raise outpatient prescriptions worth 90 billion won yearly as Dapagliflozin-based diabetes treatments. However, as a number of generics containing dapagliflozin were registered for reimbursement last month, they were subject to drug price cuts. The Ministry of Health and Welfare announced that it would cut the prices of Forxiga and Xigduo drugs by 30% from May 1 following the listing of generics. AstraZeneca Korea objected to this and filed an administrative lawsuit and at the same time applied for suspension of execution. As Forxiga and Xigduo also have indications for chronic heart failure and chronic nephropathy that have not expired patents, listing generics with only diabetes indications cannot be the basis for lowering original drug prices. It also argued that if the suspension of execution is not accepted, there is a risk of damage that is difficult to recover. If the court accepts the company's argument, the company can avoid losses of about 27 billion won a year until the prominent lawsuit is decided. In many cases, it takes more than three years from the citation of the suspension of execution to the cancellation of the drug price cut to the Supreme Court, so the company can prevent losses of tens of billions of won. However, it can be a burden that the judiciary's decision on the drug price cut enforcement suspension has been pointed out one after another it is fragmentary. In Korea's drug pricing system, which does not differentiate drug prices according to indications, questions are being raised as to whether the company's claims of inconsistency in price cuts due to inconsistency in drug prices are reasonable. This is because if the Ministry of Health and Welfare wins the prominent lawsuit after citing the suspension of execution, it will not be able to avoid criticism that the court cited the application for suspension of execution too broadly.
- Company
- HLB applies for liver cancer drug approval to FDA
- by Lee, Seok-Jun May 19, 2023 05:47am
- HLB submitted a New Drug Application (NDA) to the FDA for Rivoceranib, a targeted anti-cancer drug under development as a first-line liver cancer drug. This is the first time that a domestic bio company has completed its own clinical trials for its anti-cancer drug substance and proceeded with the new drug approval process in the global market. HLB started global clinical trials of Rivoceranib in 2011. Through its US subsidiary, Elevar Therapeutics, it has developed the combination of Rivoceranib and Camrelizumab as a first-line treatment for liver cancer. In the global phase 3 (CARES 310) study of 543 patients in 13 countries, compared to control sorafenib, 3 CR vs. 1 person, mOS 22.1 months vs. 15.2 months, mPFS 5.6 months vs. 3.7 months, ORR 25.4% vs. 5.9% was derived. It demonstrated therapeutic efficacy regardless of region (Asia vs. non-Asia) and cause (viral vs. non-viral). In particular, the Hazard Ratio was 0.62 for the overall survival period and 0.52 for the progression-free survival period, lowering the patient's risk of death by 40-50%. The FDA said at the pre-NDA (meeting before the application for new drug approval) that there was 'no problem' in the NDA submission for the Rivoceranib combination method. China already approved it as a first-line treatment for liver cancer in February this year. Rivoceranib is a TKI oral drug that effectively kills cancer by inhibiting VEGFR-2 and blocking the supply of oxygen and nutrients essential for cancer growth. To date, there is no approved first-line treatment for liver cancer that is a combination of a TKI anticancer drug with an angiogenesis inhibitory mechanism and an immune anticancer drug. HLB CEO Kim Dong-gun said, "We will do our best for the remaining procedures so that liver cancer patients and their families waiting for new treatment options, as well as employees and shareholders who have been with us on the long journey of new drug development can feel comfort and pride." On the other hand, HLB holds the global patent for Rivoceranib, HLB Life Science holds the Korean copyright and some profit rights in Europe and Japan, and Jiangsu Hengrui Medicine holds the Chinese copyright. All other global rights belong to Elevar Therapeutics.
- Company
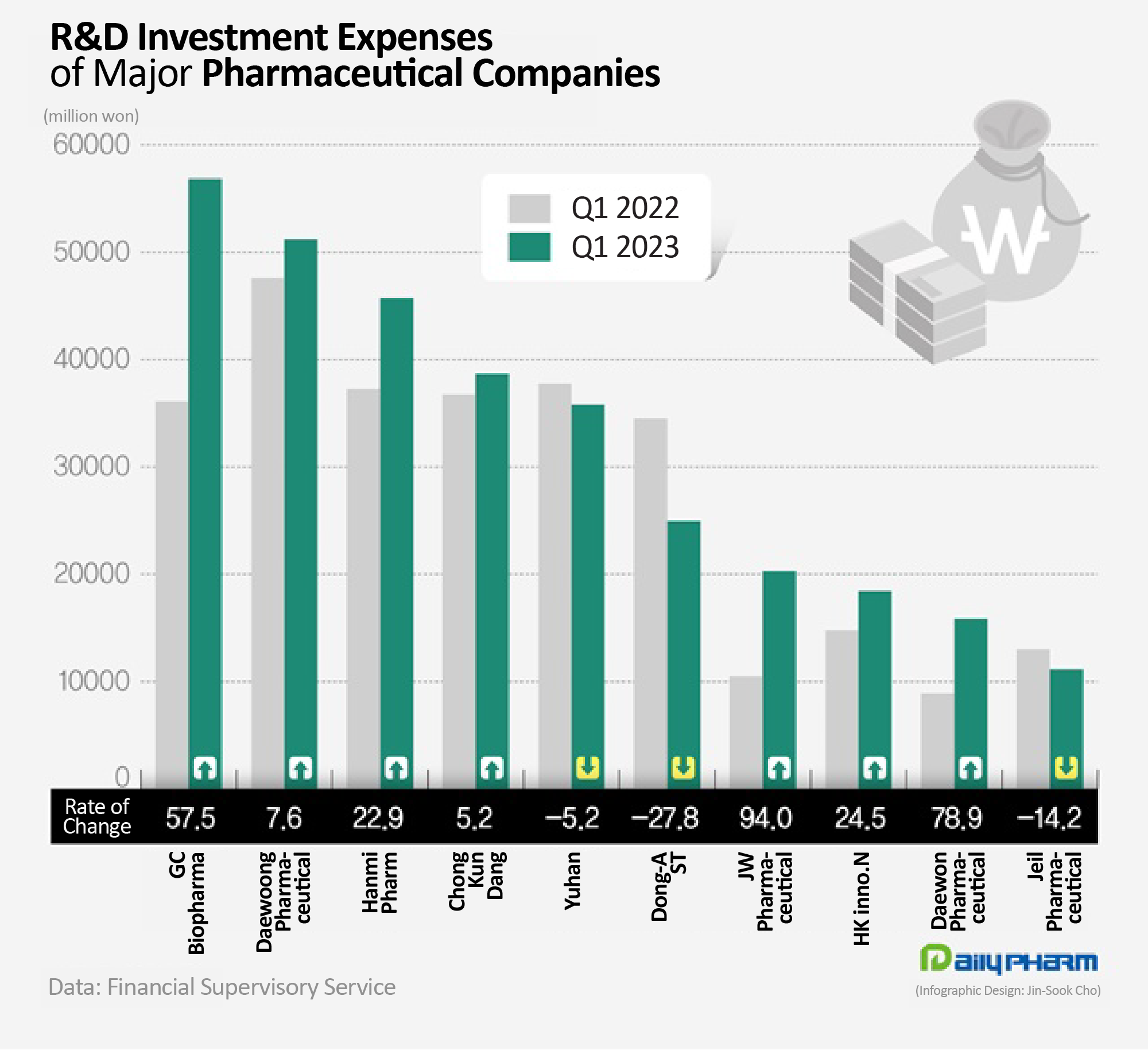
- Korean pharma industry expands R&D investments
- by Chon, Seung-Hyun May 19, 2023 05:47am
- Pharmaceutical companies have vigorously engaged in research and development (R&D) activities to discover future foods. 7 out of 10 major traditional pharmaceutical companies increased their R&D investment compared to last year. R&D expenditures have increased significantly due to the development of new drugs and the introduction of new R&D pipelines. In particular, R&D investments by GC Biopharma and JW Pharmaceuticals soared. According to the Financial Supervisory Service on the 25th, the R&D investment made by 20 major biopharmaceutical companies in Q1 totaled up to KRW 390.6 billion, which was 13.4% increase from the previous year. The top 20 sales of traditional pharmaceutical companies that submitted quarterly reports were counted. 14 out of 20 major pharmaceutical companies saw an increase in their R&D investment expense in Q1 this year compared to last year. Among traditional pharmaceutical companies in Korea, GC Biopharma was found to have spent the most on R&D. GC Biopharma’s R&D expenses in Q1 amounted to KRW 56.9 billion, which is a 57.5% YoY increase. GC Biopharma’s R&D expenditure increased because it recently introduced a new pipeline from a foreign company. In February, GC Biopharma signed an Asset Purchase Agreement with Catalyst Biosciences, a US new drug developer, for a pipeline related to rare blood clotting disorder. GC Biopharma acquired a total of 3 programs, including ‘Marzeptacog alfa (MarzAA)’ that the company is developing in the Global Phase III trial. In March, GC Biopharma exercised its license option to the Canadian company, Acuitas Therapeutics, for its lipid nanoparticle (LNP) delivery system to develop a messenger RNA (mRNA)-based therapeutics. LNP is a delivery system that safely transports nanoparticles to cells in the body to enable mRNA to function. The ratio of R&D investment to sales at GC Biopharma increased twofold from 8.7% to 16.3% in one year. Daewoong Pharmaceutical's sales increased 7.6% YoY to reach KRW 51 billion in Q1 this year. Daewoong Pharmaceutical is currently developing new drugs for ulcerative colitis, idiopathic pulmonary fibrosis, obesity, autoimmune diseases, and infectious diseases. It is also conducting joint research with HanAll Biopharma, Daewoong Therapeutics, Oncocross, and D&D Pharmatech. Daewoong Pharmaceutical received approval for its gastroesophageal reflux disease treatment Fexclu in 2021 and succeeded in commercializing Envlo, a new diabetes SGLT-2 inhibitor class drug last year. Hanmi Pharmaceutical’s R&D investment also increased 22.9% YoY to reach KRW 45.7 billion in Q1 this year. Hanmi Pharmaceutical is developing new drugs for nonalcoholic steatohepatitis and idiopathic pulmonary fibrosis in the field of new biological agents. The company is also developing new combination drugs for diabetes and antithrombosis. Among major pharmaceutical companies, JW Pharmaceutical’s R&D expenditure increased the greatest. JW Pharmaceutical’s Q1 R&D expenditure was KRW 20.3 billion, increasing 94.0% YoY from the KRW 10.5 billion in Q1 previous year. JW Pharmaceutical started a Phase III clinical trial of 'URC-102', a gout treatment, at the end of last year. URC-102 is a uric acid excretion promoter that inhibits Urate transporter 1 (URAT)-1, which allows uric acid to be absorbed back into the body. It is effective for gout disease caused by hyperuricemia in which the concentration of uric acid in the blood is abnormally high. The Phase III trial will compare URC-102 with a total of 588 gout patients with the existing treatment febuxostat. Daewon Pharmaceutical and Handok’s Q1 R&D investment increased 50% from the previous year. R&D expenditures increased by over 20% at Hugel, HK Inno.N, and Dong Wha Pharm. On the other hand, the R&D investment amount of companies including Dong-A ST, Il-Yang Pharmacuetical, Jeil Pharmaceutical, Boryung Pharmaceutical, United Pharm, and Yuhan Corp decreased YoY. In terms of the R&D-to-sales ratio, Daewoong Pharmaceutical’s rate was highest at 17.5%. GC Biopharma, Dong-A ST, Daewon Pharmaceutical, Hanmi Pharmaceutical, JW Pharmaceutical, United Pharm, Samjin Pharm, Chong Kun Dang, and Ilyang Pharm invested more than 10% of their sales in R&D.
- Company
- 90% of pricing managers unsatisfied with new drug price
- by Eo, Yun-Ho May 19, 2023 05:46am
- Study results showed that about 90% of the drug pricing managers in Korea are not satisfied with the value recognized for new drugs. Recently, a study on ‘'An Industry Survey on Unmet Needs in South Korea’s New Drug Listing System' was published on the online version of the medical science journal Springer (https://link.springer.com/article/10.1007/s43441-023-00531-3). 6 authors including Professor Jong-Hyuk Lee of the Chung-Ang University College of Pharmacy, expert advisory member Sungju Kim from Lee&Ko participated in the study. The study was conducted with the cooperation of 3 industry associations: Korea Pharmaceutical and Bio-Pharma Manufacturers Association, the Korean Research-based Pharmaceutical Industry Association, and the Korea Biomedicine Industry Association. Members of the associations that in charge of insurance drug pricing participated in the study. The total number of respondents was 56, 34% of which were from domestic companies and 66% from multinational pharmaceutical companies. The survey consisted of questions that study the industry's satisfaction with the current insurance system, requests for improvements in the new drug reimbursement listing system, including the pharmacoeconomic evaluation system, pharmacoeconomic evaluation exemption system, and risk-sharing agreement scheme, and the need to introduce systems that have not been introduced to Korea. According to the results, 64.3% and 89.3% of respondents answered that they were dissatisfied with the patient accessibility and value recognition of new drugs, respectively, and answered that institutional improvement for rare diseases was needed the most (41.1%). Regarding the pharmacoeconomic evaluation system, 92.9% said that the ICER threshold needs to be improved. In the risk-sharing system, the reimbursement standard expansion system required improvement (91.1%), and in the risk-sharing agreement scheme, the expansion of target diseases (89.3%) was needed. Also, regarding the reimbursement listing of anticancer drugs, 83.9% answered that the Cancer Disease Review Committee, which determines the reimbursement standards, needs to be improved. In the case of general drugs, the majority of respondents said that the drug price negotiation system needs to be improved. When asked about the need to introduce a system that is yet to be introduced, respondents expressed a high need to introduce a drug pricing system by indication and insisted that the system should be introduced regardless of the severity of the disease, whereas the pre-listing post-evaluation system should be limitedly applied to life-threatening diseases. Sungju Kim said, “In general, study results showed low satisfaction with the current system and a great need for its improvement. As pharmaceutical companies are also important stakeholders, their opinions should also be considered in the process of pricing and reimbursement policy reforms.”
- Company
- Ibrance emerges as a new drug partner for metastatic breast
- by Jung, Sae-Im May 19, 2023 05:46am
- Professor Joo-Hyeok Son, Department of Oncology, Yonsei Cancer Hospital CDK4/6 inhibitor Ibrance is emerging as a combination partner for metastatic breast cancer drug developers based on its long-accumulated treatment experience. Even if the dose is increased, there is little concern about side effects, so it is expected that it will be used as a variety of combination drugs. Ibrance is the first CDK4/6 inhibitor developed by Pfizer and was launched in Korea in the fourth quarter of 2016. It provided a new treatment option for patients with HR+/HER2- metastatic breast cancer who had to use anti-hormonal drugs such as aromatase inhibitors or chemotherapy if not managed with these drugs. Ibrance, which held the top spot in the CDK4/6 market for five years, faced a decline for the first time last year. According to IQVIA, a pharmaceutical market research institute, Ibrance sales decreased 14% from 65.6 billion won in 2021 to 56.2 billion won last year. The rapid growth of generics has affected Ibrance's sales. Generics such as Verzenio and Kisqali are breaking down Ibrance's dominance through more sophisticated clinical trials and new field development. In particular, with generic drugs expanding their scope to early breast cancer, prospects are raised that Ibrance's position, which is limited to metastatic breast cancer, will narrow. In this situation, Iran is trying to turn around by emerging as a new drug combination partner for metastatic breast cancer. In an interview with Daily Pharm, Professor Sohn Joo-hyeok of the Department of Oncology at Yonsei Cancer Hospital said, "To use an oral SERD instead of an aromatase inhibitor, it must be used in combination with CDK4/6, but Irance is widely adopted as a combination drug." It means being recognized." Recently, oral SERD development is in full swing as a CDK4/6 inhibitor combination therapy for breast cancer. Many global pharmaceutical companies such as Pfizer, AstraZeneca, and Menarini jumped into the market. Big pharma is in the midst of studying combination therapy with CDK4/6 inhibitors as well as oral SERD monotherapy, and most of them chose Ibrance as the combination drug. Professor Sohn cited safety as the reason why Ibrance received much love calls as a combination drug. Professor Sohn said, "Ibrance's strength is that it is stable as it has accumulated the longest treatment experience among CDK4/6 inhibitors." It's less, so I'll consider it first," he explained. This evaluation was proven with real-world data. The Ibrance P-REALITY X study conducted by Pfizer is a large-scale real-world study that retrospectively analyzed the data of 2888 patients with HR+/HER2- metastatic breast cancer enrolled from February 2015 to March 2020. Patients receiving Ibrance plus Letrozole combination therapy as a first-line treatment option were compared with patients on Letrozole monotherapy. As a result of matching the baseline characteristics of the two groups similarly, the median overall survival (mOS) of the Ibrance group was 49.1 months, which was significantly prolonged compared to 43.2 months of the Letrozole single group, reducing the risk of death by 24%. It is less toxic, so even elderly patients can use it without burden. As a result of a sub-analysis examining Ibrance and Letrozole combination therapy in elderly patients aged 65 years or older, the median progression-free survival (mPFS) was 30.6 months, compared to 19.1 months in the control group. Professor Sohn said, "Recently, I prescribed Ibrance to an 80-year-old elderly patient with an anti-hormone drug. The treatment went well, and people around me said, 'How can you correct this when you are receiving chemotherapy at an advanced age?' I always worry about prescribing medications for cancer, but Ibrance greatly eases that burden."
- Company
- Chong Kun Dang has the domestic license for the 110 billion
- by Chon, Seung-Hyun May 18, 2023 05:44am
- Chong Kun Dang bought the domestic license for MSD’s blockbuster diabetes treatment ‘Januvia series’. It is equipped with a stable cash cow that raises more than 100 billion won a year. Chong Kun Dang signed a license agreement with MSD headquarters in Switzerland to introduce all domestic rights for three diabetes treatments, Januvia, Janumet, and Janumet XR. Chong Kun Dang acquires not only domestic sales and distribution rights for the three Januvia series, but also all rights such as licenses, trademarks, and manufacturing. The contract period is from July 15th to August 31st, 2038. The total contract amount is 45.5 billion won. Chong Kun Dang paid MSD headquarters a down payment of 23 billion won, and the milestone scale according to sales is 17 million dollars (approximately 22.5 billion won). But Since 2016, Chong Kun Dang has jointly sold the Januvia series with MSD Korea. Through this contract, it exclusively secured domestic rights for the Januvia series for the next 15 years. Chong Kun Dang receives the Januvia series from MSD headquarters and sells them exclusively in Korea. Januvia is a DPP-4 inhibitory antidiabetic drug containing Sitagliptin. Janumet is a combination drug combining Januvia and metformin. According to UBIST, a pharmaceutical research institute, the Januvia series jointly invested a total of 109.4 billion won in outpatient prescriptions last year. Januvia and Janumet raised 40.5 billion won and 68.9 billion won, respectively. Outpatient prescription amount of Januvia series by year (unit: KRW 100 million, source: UBIST) The amount of prescriptions for the Januvia series last year decreased from 130.7 billion won in 2020 and 124.6 billion won in 2021, which is the aftermath of drug price cuts. In March of last year, through a 'trade-off' agreement with the government, MSD lowered the insurance cap for the Januvia series by an average of 6.0%. The drug price of the Januvia series was voluntarily lowered as a condition of expanding reimbursement for Keytruda, an immuno-oncology drug. Considering the drug price reduction rate of the Januvia series, it means that it still has a great influence in the market. As Chong Kun Dang has secured all licenses for the Januvia series, profitability from future sales is expected to increase. An official from Chong Kun Dang said, “We were leading the market with a diverse portfolio of diabetes treatments, including Duvie,” and “We stably expanded the treatment options for patients by securing the Januvia series.”
- Company
- Benefit extended DM Drug
- by Moon, sung-ho May 17, 2023 05:38am
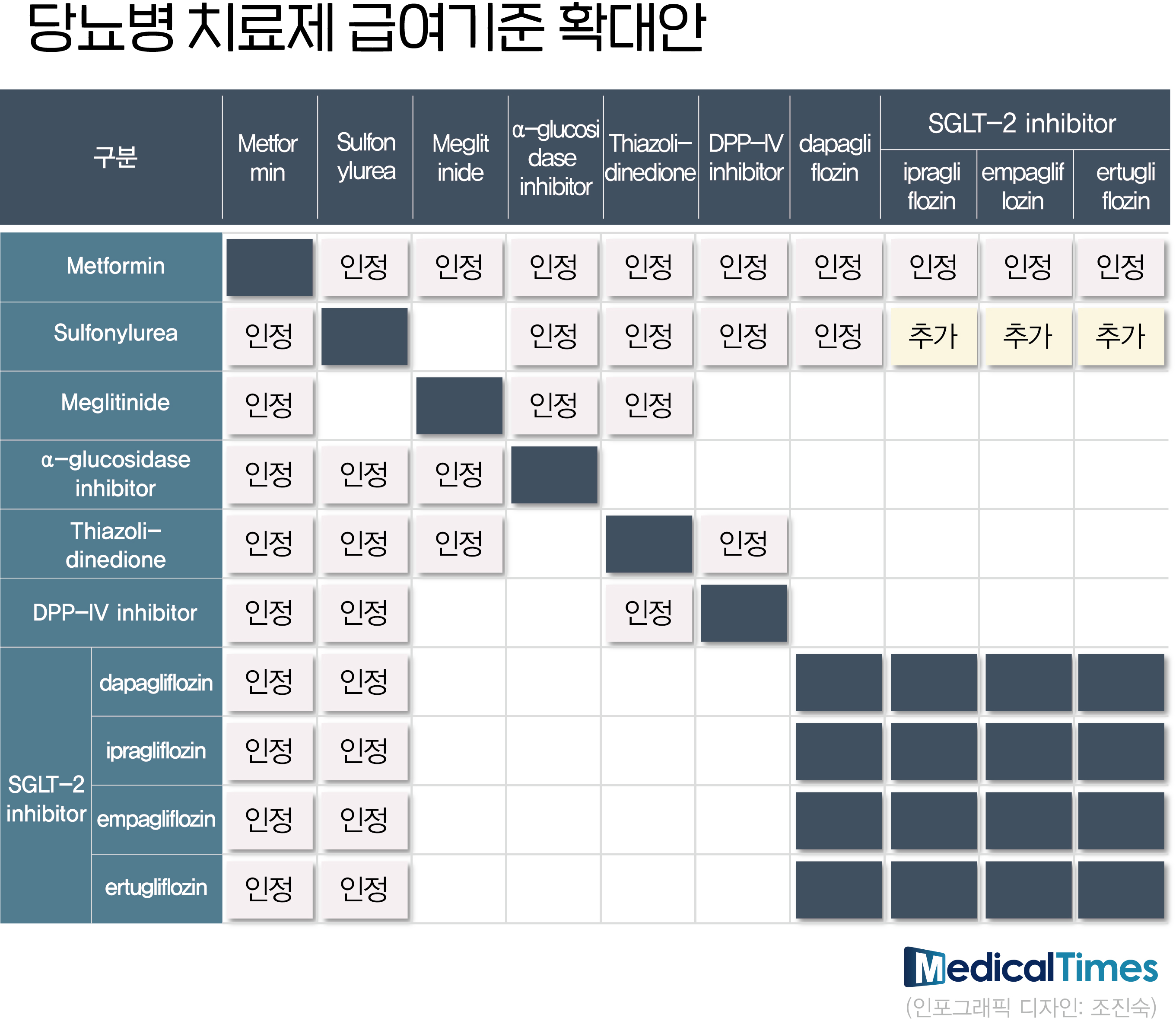
- Clinical sites are busy finding the optimal prescription combination while the expansion of diabetes treatment reimbursement standards for each class and the release of generics following the patent expiration of original items coincided. It is an effort to find the optimal combination for each treatment category that can be covered by health insurance to minimize patient burden. According to the pharmaceutical industry on the 26th, the Ministry of Health and Welfare significantly eased the criteria for the accreditation of diabetes medications this month. The key is that various drug combinations are possible without specifying the SGLT-2 inhibitor component. With this revision, SGLT-2 inhibitors such as Ipragliflozin, Empagliflozin, and Ertugliflozin can be reimbursed when used together. Combinations of Metformin + SGLT-2 inhibitor + DPP-4 inhibitor and Metformin + SGLT-2 inhibitor + TDZ combination are also acceptable if the HbA1C is 7% or higher even if the two-drug regimen is administered for more than 2 to 4 months. Insurance cannot be provided if only SGLT-2 inhibitor + DPP-4 inhibitor or TZD is used without Metformin. In the clinical field, following the release of Forxiga's generic products in April, guidance is being given to prescribing DPP-4 inhibitors or TZD with reimbursement instead of prescribing inexpensive SGLT-2 inhibitors through the full cost of the patient's expenses. This is because the 2nd union is excluded from the benefit target. A professor of endocrinology at A University Hospital, an executive officer of the Korean Diabetes Association, said, "Three-drug therapy was applied as reimbursement, but SGLT-2 inhibitor + DPP-4 inhibitor or TZD two-drug therapy is not reimbursed, so it can be a prescription form." It is still in the early stages of expanding the salary standard, so there are various opinions coming and going.” In addition, there is an opinion that there may be a situation where there is no choice but to recommend the patient to take without 'metformin' instead of prescribing the three-drug therapy as the two-drug regimen is not covered. In the case of patients who cannot take metformin due to side effects, SGLT-2 inhibitor + DPP-4 inhibitor or TZD two-drug therapy is a prescription pattern that can occur in clinical settings because reimbursement is not possible. As the two-drug regimen is not possible, instead of prescribing SGLT-2 inhibitors as non-covered drugs, it is possible to recommend taking metformin out of the three-drug regimen that can be covered. In the pharmaceutical industry, it is a phenomenon that can occur in clinical settings, but it is evaluated that it will be only a small part. An executive of a domestic company A, who used to be a doctor, said, “It is a prescription pattern that can happen at the moment, but it is a concern raised because of changes in the clinical field due to the expansion of the reimbursement standard have not yet taken place.” The Ministry of Health and Welfare plans to list SGLT-2 inhibitors and DPP-4 inhibitor complexes of major pharmaceutical companies in May, following the expansion of the reimbursement standard and the Forshiga generic this month. Specifically, ▲AstraZeneca Qtern, ▲Boehringer Ingelheim's Esgliteo, ▲MSD Stegluzan, and ▲LG Chem's Zemidapa are included. In addition, Daewoong Pharmaceutical's Envlo, a new diabetes drug in the domestic SGLT-2 inhibitor class, will enter the prescription market in earnest with reimbursement.
- Company
- SK Bioscience & MSD signed a consignment production contract
- by Jung, Sae-Im May 17, 2023 05:38am
- At the contract signing ceremony held in Jongno-gu, Seoul, government officials such as Second Vice Minister of Health and Welfare Park Min-soo, MSD Vice President Sanat Chattopadhyay, Hilleman Research Center CEO Raman Rao, SK Discovery Vice Chairman Choi Chang-won, SK Bioscience President Ahn Jae-yong, Hoon Kim, CEO of Global R&BD, etc. attended. MSD is developing a next-generation Zaire Ebola vaccine candidate with improved process efficiency and stability of the currently approved and used Zaire Ebola vaccine Ervebo with the Hillemann Institute, an international non-profit research institute. In the future, if the candidate substance is successfully developed and approved by regulatory authorities, it is expected to contribute to increasing the global supply and improving accessibility of the Zaire Ebola virus vaccine. The candidate material will be produced at Andong L House after SK Bioscience has transferred related development and technology, and will be supplied to international organizations after obtaining approval from relevant health authorities to be used in the management of Ebola virus disease. Ebola virus disease is a serious hemorrhagic fever disease caused by infection with the Ebola virus. The main cause of outbreaks in the past 20 years has been the Zaire Ebola virus. Since the Ebola virus was first discovered in 1976, several outbreaks have caused serious human and economic damage. Starting with this contract, SK Bioscience plans to expand its CMO and CDMO business in earnest. Based on R&D technology proven with various self-developed vaccines and state-of-the-art vaccine production facilities, it is a strategy to expand the C(D)MO business for various infectious diseases to respond quickly to new pandemics and take the lead in promoting public health. In addition to the existing vaccine platform, C(D)MO business for new platforms such as mRNA and CGT will also be promoted. A pilot plant that will enhance competitiveness in the C(D)MO market will be built in the ‘Global R&PD Center’ established in Songdo, Incheon through the largest facility investment by SK Bioscience after its launch. The pilot plant, which is a small-scale test facility built before introducing new methods or products, will be equipped with facilities that can carry out research tasks such as CGT, mRNA, and viral vectors. Vice Chairman Choi Chang-won of SK Discovery said, "This collaboration is the result of SK bioscience's production capacity and global network recognized through COVID-19, and it will be an important milestone in our efforts to contribute to the promotion of human health." We hope that the cooperation between SK, MSD, and Hilleman Laboratories will be further expanded with the government, such as the Ministry of Health and Welfare and the Korea Centers for Disease Control and Prevention, based on the common belief that it will solve the problem of imbalance in the country's vaccine supply and expand access to vaccines."