- LOGIN
- MemberShip
- 2025-12-23 15:15:23
- Company
- Reimb discussions imminent for Obizur in Korea
- by Eo, Yun-Ho Jul 27, 2023 05:40am
- Takeda Pharmaceuticals Korea is quickening its steps to receive reimbursement listing for ‘Obizur,’ its treatment for bleeding episodes in patients with acquired hemophilia A (AHA). According to industry sources, Takeda Pharmaceuticals Korea’s bleeding treatment for adults with acquired hemophilia A may soon be introduced for deliberation at the Health Insurance Review and Assessment Service’s Drug Reimbursement Evaluation Committee meeting in August. As reimbursement evaluations for Obizur have been ongoing immediately after receiving approval in March, the reimbursement process is progressing relatively quickly. Obizur has been designated as an orphan drug in Korea in July 2021. Unlike existing bypass factor drugs, this drug replaces blood coagulation factor VIII with AHA indications. As a gene recombinant therapy that was produced by deleting the B-domain from a porcine factor VIII that is comparable to human Factor VIII, inhibitory antibodies do not readily recognize the treatment. Therefore, Obizur can replace the inactivated human coagulation factor VIII to help blood clotting and control bleeding. The unique mechanism of action allows Obizur to be the only AHA drug that can stably monitor patients’ blood factor VIII levels using standard assays, enabling individually tailored dosing. In a prospective, non-randomized, open-label Phase II/III study of 28 patients with AHA that evaluated the safety and efficacy of Obizur, all patients that were treated with Obizur had a positive response to treatment at 24 hours after initial dosing. A positive response was one where the bleeding had stopped or was reduced, with clinical improvement or with factor VIII activity above a pre-specified target. The treatment success rate after administration of the final dose (within 2 weeks of administration) was 85.7% (24/28), and the rate was higher in patients treated with Obizur as first-line therapy. The treatment success rate was 94% (16/17) in patients treated with Obizur as first-line therapy, and 73% (8/11) in patients treated with Obizur as second-line therapy. No serious adverse events or deaths were observed with Obizur in the study.
- Company
- Astellas launches urothelial cancer drug Padcev
- by Kim, Jin-Gu Jul 26, 2023 05:41am
- Astellas officially launched 'Padcev' in Korea as a drug to treat locally advanced or metastatic urothelial cancer. Astellas Pharmaceutical Korea held a press conference to commemorate the launch of Padcev in Korea at the Intercontinental Hotel in Seoul on the 19th. Urethral carcinoma is a type of bladder cancer. It is estimated that 90% of bladder cancers are urothelial carcinomas. The survival prognosis is not known to be good. Non-muscle invasive bladder cancer that does not invade the bladder muscle has a 5-year relative survival rate of 80%. However, if the cancer invades the bladder muscle, the 5-year relative survival rate drops sharply to 50%. In particular, about half of them lead to distant metastasis. At this time, the 5-year relative survival rate is further reduced to 5%. Patients receive platinum-based chemotherapy as first-line treatment and PD-1 or PD-L1 inhibitors as second-line treatment. However, in the case of platinum-based chemotherapy, which is the first-line treatment, low tolerance and high possibility of disease progression were cited as problems. Immunotherapy that can be considered as a second-line treatment showed that only 13-28% of patients responded to treatment regardless of PD-L1 status. As a result, most of the disease progressed within 3 months of treatment. Padcev is a treatment that can be used for patients whose cancer has progressed or relapsed after chemotherapy (first-line treatment) and immuno-anticancer drugs (second-line or first-line maintenance therapy) in situations where treatment options are not feasible. Padcev is an antibody-drug conjugate (ADC) that targets nectin-4. In March of this year, it was approved as monotherapy by the Ministry of Food and Drug Safety. It is first recommended as Category 1 in the NCCN Guidelines. The Padcev phase 3 trial was conducted by comparing and evaluating Padcev with existing chemotherapy drugs in 608 patients with locally advanced or metastatic urothelial cancer. As a result, it was found that the Padcev administration group reduced the risk of death by about 30% compared to conventional chemotherapy. The median overall survival (OS) of the Padcev-administered group was 12.9 months, which was 3.97 months longer than the chemotherapy group, demonstrating a significant improvement in survival time. In addition, the median PFS of the Padcev-administered group was 5.6 months and that of the control group was 3.7 months, indicating a 37% reduction in the risk of disease progression. Kim Mi-so, a professor of oncology at Seoul National University Hospital, said, "Platinum-based chemotherapy is mainly based on cisplatin. However, the PFS ranges from 7.7 to 9.5 months, so most of the disease progresses within a year." Professor Mi-So Kim said, “Second-line PD-1/L1 inhibitors are recommended for patients whose disease has progressed after the first-line platinum-based chemotherapy, but most patients do not respond to treatment.” Park Kyung-ah, director of Astellas Pharmaceuticals Korea, said, "We will operate the patient program until Padcev is covered by reimbursement." Kim Joon-il, CEO of Astellas Pharmaceuticals Korea, said, “We are delighted to be able to release Padcev as early as possible.” "With Padcev, we will be able to provide innovative treatment options to patients with locally advanced or metastatic urothelial cancer in Korea," he said.
- Company
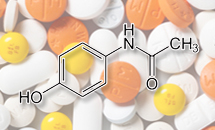
- Acetaminophen prescriptions at a record high
- by Chon, Seung-Hyun Jul 26, 2023 05:41am
- The single-agent antipyretic analgesic ‘acetaminophen’ recorded the largest-ever sales in outpatient prescriptions. The prescription market for acetaminophen has increased significantly due to the unceasing number of confirmed COVID-19 cases arising despite the end of the COVID-19 pandemic and the increase in flu and cold patients. The rise in the insurance drug price of acetaminophen the government made to stabilize acetaminophen supply has also contributed to the market expansion. According to the market research institution UBIST, the outpatient prescription market for single-agent acetaminophen drugs in Q2 was KRW 14.1 billion, up 89.2% YoY. The increase in the prescription amount was 5.5% YoY to KRW 11.8 billion in Q1, which grew further in Q2. The acetaminophen market broke its record for 2 consecutive quarters in Q2. In the first half of the year, acetaminophen prescriptions rose to KRW 26 billion, up 38.9 % from the same period last year. The acetaminophen prescription market had fallen to the KRW 4 billion-5 billion range by Q3 2021 after recording KRW 9.2 billion in Q4 2019 and KRW 7.8 billion in Q1 2020. At the time, COVID-19 was considered the direct cause of the contraction of the acetaminophen prescription market. The stricter personal hygiene practices that followed the spread of COVID-19, including washing hands and wearing masks, had greatly reduced cases of infectious diseases such as flu and colds, thus contracting relevant treatment markets. The acetaminophen prescription market rebound to KRW 6.7 billion in Q4 2021 and then soared to KRW 11.2 billion in Q1 last year. At the beginning of last year, the demand for acetaminophen increased significantly with hundreds of thousands of COVID-19 cases being confirmed a day. Then, prescriptions decreased to KRW 7.5 billion and KRW 8.2 billion in the Q2 and Q3 of last year, respectively. However, it made a rebound again to KRW 10.9 billion in the Q4 of last year and continued on its rise this year. The industry analysis is that the acetaminophen prescription market has grown further as the number of confirmed COVID-19 cases continues to occur by tens of thousands even after the end of the pandemic, and the number of flu or cold patients has also increased after the mandatory mask-wearing regulation was lifted this year. Quarterly outpatient prescriptions of acetaminophen single-agent drugs (Unit: KRW 100 million, Data: UBIST) The number of flu patients has continued to exceed the epidemic standard from the beginning of this year to June. According to the Korea Disease Control and Prevention Agency, the number of suspected influenza patients per 1,000 outpatients dropped from 52.5 in Week 1 of 2023 to 11.7 in March. However, the number of suspected flu cases rose again from April. For 6 consecutive weeks from the 17th week (fourth week of April) to the 22nd week last week of May), the number of suspected influenza patients per 1,000 outpatients continued to be over 20. This greatly exceeds the flu epidemic standard of 4.9 set by the KDCA. The raised insurance drug price of acetaminophen was also pointed out as another factor that contributed to the expansion of its prescription market. The Ministry of Health and Welfare raised the insurance price ceiling of 18 acetaminophen 650mg items by up to 76.5% since December last year. The insurance price limit for 650mg acetaminophen was only KRW 43 to 51 before then but was raised to KRW 90. The government made an unprecedented decision to raise the price of all acetaminophen together when pharmaceutical companies expressed reluctance to increase production due to the drug’s poor cost structure. However, it is a temporary increase that will be adjusted to KRW 70 from December this year. Affected pharmaceutical companies had promised to increase production of acetaminophen in line with the price hike. The upper ceiling price for acetaminophen drugs was determined through negotiations between pharmaceutical companies and the National Health Insurance Service based on manufacturing/import costs and factors that render the price increase necessary, production/import volume, etc. Janssen Korea’s Tylenol 8 Hour received the highest price hike of 76.5%, from KRW 51 to KRW 90. Prices of Bukwang Pharm’s Tacenol 8 Hours and Chong Kun Dang's Penzal were each raised from KRW 51 to KRW 88, a 72.5% hike. Price of Hanmi Pharm’s Suspen 8 Hours increased by 70% from KRW 50 to KRW 85. The price of Kolon Pharmaceutical's Tramol rose 66.7% from KRW 51 to KRW 85, while Genu Pharma’s Anispen 8 Hour and Hana Pharm's Tylicol 8 Hour rose 62.7% each to become KRW 83. The price of Sama Pharm’s Setopen and Yoong Poong Pharmaceutical’s Tifen 8 Hours rose 56.9% each to become KRW 80 from KRW 51. Also, 8 items including Boryung Biopharma’s Cetaphen 8hr were raised to the KRW 70 range. The price increase of acetaminophen resolved the unstable supply issue, and the increase in cold and flu patients has led to an unprecedented boom in the acetaminophen prescription market.
- Company
- MSD Korea appoints Albert Kim as new managing director
- by Eo, Yun-Ho Jul 26, 2023 05:41am
- General Manager Albert Kim On the 25th, MSD Korea announced that it has appointed Albert Kim (pic) as the new managing director, effective August 1st. The new managing director Kim is a seasoned pharma and bio expert who has served in global and Korean pharmaceutical bio companies for more than 25 years, accumulating extensive experience in local and global markets, including Korea. Kim has majored in Biochemistry at McMaster University and acquired an MBA at Schulich School of Business at York University, Canada. In various leadership positions, Kim led teams and groups in various countries and regions other than Korea, including the U.S., EU, Canada, Brazil, Switzerland, Sweden, Thailand, Singapore, Malaysia, and Taiwan. Before being appointed as a managing director of MSD, Kim served as the Vice President of the Commercial Division at Samsung Bioepis, where he oversaw the launch and growth of key major product portfolios in the U.S. and EU, in charge of global commercial strategy and operations. Kim had also served as the Chief Financial Officer (CFO) at Novartis, and as the founding General Manager of Menarini Korea where he led the establishment and growth of the Korean branch. GM Albert Kim said, “We will continue to live true to MSD's long-standing mission of saving and improving lives by improving medical access to MSD Korea's innovative medicines, vaccines, and pipelines.”
- Company
- Two types of JAK inhibitors start a full-scale competition
- by Eo, Yun-Ho Jul 26, 2023 05:40am
- The competition between two types of JAK inhibitors in the field of juvenile atopic dermatitis has begun in earnest. Following AbbVie Korea's Rinvoq last April, Pfizer Korea's Cibinqo was covered by insurance for the indication of atopic dermatitis in children and adolescents from this month (July). Cibinqo stopped the reimbursement process after passing the Pharmaceutical Reimbursement Criteria Subcommittee of the Health Insurance Review and Assessment Service in August last year, and at the beginning of this year, it expanded the scope from adults to teenagers over 12 years of age and resubmitted the application for reimbursement. After passing the Pharmaceutical Reimbursement Evaluation Committee in March, the drug price negotiations were concluded with HIRA in June. In terms of atopic dermatitis as a whole, the competition for JAK inhibitors is a three-way battle, but Lilly Korea's Olumiant has no indications for children and adolescents. Therefore, Rinvoq, Cibinqo, and Sanofi Korea's Dupixent, an interleukin drug, are expected to compete in this area. However, as Dupixent is relatively expensive and there are detailed differences in indications, competition among JAK inhibitors is expected to be fiercer. The prescription price of Rinvoq15mg for severe atopic adolescents aged 12 to 17 is 59,493 won, and the prescription cost of Cibinqo for the same age group is 53,217 won. The case of adults is similar. Rinvoq 30mg is 94,884 won and Cibinqo 200mg is 77,826 won, which is a similar drug price. The general hospital prescription environment is also stable on both sides. Both Rinvoq and Cibinqo have passed drug committees (DCs) of major medical institutions nationwide, including the big five tertiary hospitals, including Samsung Seoul Hospital, Seoul National University Hospital, Seoul St. Mary's Hospital, Asan Medical Center, and Shinchon Severance Hospital. In the case of Rinvoq, the high-dose (30mg) formulation has also been coded at 30 medical institutions, including Seoul Asan Medical Center, Shinchon Severance Hospital, Seoul St. Mary's Hospital, and the National Medical Center. The two drugs can be applied for insurance coverage among adults (18 years of age or older) and adolescents (12 to 17 years of age) patients with chronic severe atopic dermatitis who have symptoms lasting for more than 3 years ▲ who are not properly controlled even after administering a topical treatment for 4 weeks or more as the first treatment, ▲ there is no response or cannot be used due to side effects, etc. even after administering systemic immunosuppressants for more than 3 months, ▲ and ▲ have an EASI of 23 or higher before starting administration.
- Company
- Decreased drug price and inclusion of impurities in Januvia
- by Jung, Sae-Im Jul 25, 2023 05:46am
- Nitrosamine impurities management hidden ambush... The standard will be significantly strengthened. The diabetes treatment 'Januvia series', for which sales rights were recently transferred to Chong Kun Dang, is experiencing sluggishness in the prescription market. Following last year's drug price cut, the company suffered voluntary withdrawals due to excess impurities in the first half of this year, and the scale shrank by 13% in two years. According to UBIST, a pharmaceutical market research institute, on the 24th, the total outpatient prescriptions for the Januvia series (Januvia, Janumet, and Janumet XR) in the first half of this year were 75.4 billion won, down 8% from 81.9 billion won in the same period last year. By item, Janumet decreased by 9% from 36.2 billion won in the first half of last year to 32.9 billion won this year. Then, Januvia 10% (21.7 billion → 19.6 billion won) and Janumet XR 5% (24 billion → 22.9 billion won) each decreased. Sitagliptin is a treatment for type 2 diabetes. It is a representative DPP-4 inhibitory mechanism that led the domestic diabetes market. The Januvia series consists of a total of three products. ▲Sitagliptin single drug 'Januvia' ▲Metformin + Sitagliptin combination drug 'Janumet' ▲Janumet extended-release formulation 'Janumet XR' with enhanced convenience. Janumet, Janumet XR, and Januvia have the highest prescriptions in that order. MSD opened the DPP-4 inhibitor market with the domestic approval of Januvia and Janumet in 2007, and in 2013, it had a lineup with Janumet XR. DPP-4 inhibitors have become mainstream by replacing existing diabetes drugs with the advantage of having a good blood sugar-lowering effect and little concern for side effects. Among them, the Januvia series has emerged as the most widely used DPP-4 inhibitor in Korea. It is 2021 when prescriptions for the Januvia series reach their peak. The Januvia series, which recorded 164.3 billion won in 2019 and 173.8 billion won in 2020, raised 176.3 billion won in 2021. Janumet 79.2 billion won, Janumet XR 50 billion won, Januvia 47.1 billion won. In particular, the number of outpatient prescriptions recorded by the three products in the second half of 2021 was 89.4 billion won, the largest half-year prescription amount ever. The decline of the Januvia series started last year. The biggest factor is the drug price of the Januvia series. MSD has signed a 'trade-off' agreement with the government to expand reimbursement for Keytruda, an immuno-oncology drug that it has been pushing for. It is content that voluntarily lowers the price of the Januvia series in exchange for allowing the expansion of the primary lung cancer benefit for Keytruda. With this agreement, the prices of all three Januvia products have been lowered by an average of 6% since March last year. In the aftermath of drug price cuts, last year's Januvia series recorded 81.9 billion won in the first half and 80.6 billion won in the second half, down 6% and 10% year-on-year. In the first half of this year, the decline was even greater with an 8% decline. It is analyzed that there was an additional drug price cut of about 1% due to the expansion of insurance coverage for diabetes drugs and that some products were affected by voluntary recalls due to the occurrence of impurities that exceeded the standard. Compared to two years ago, the amount of prescriptions has decreased by 13%. In the second half of this year, the decline is expected to continue due to the expiration of the Januvia patent. The Januvia substance patent expires on September 1. Domestic pharmaceutical companies are preparing to release sitagliptin generics in time for expiration. If a generic with the same ingredients is registered for reimbursement, the price of Januvia is automatically reduced by 30%. A simple calculation of drug price cuts would result in about 50 billion won of annual prescriptions being subtracted. Attention is focused on the move of Chong Kun Dang, which acquired the Januvia series from MSD. Chong Kun Dang paid 45.5 billion won (down payment + milestone) to acquire all rights, including license, trademark, manufacturing, sales, and distribution of Januvia. Considering the annual prescription amount of the existing Januvia series, 45.5 billion won is an amount that can be recovered within half a year. However, considering drug price cuts and competition among generics, Chong Kun Dang is bound to be nervous. Some predict that the key to Januvia's future generic competition will be impurities. Januvia carries the risk of nitrosamine-like impurities, and the tentative daily intake is set at 246.7 ng. The global guidelines aim to lower the daily allowance to 37ng in the future. It is expected that the stricter standards will be applied at the end of the year or next year at the earliest. To this end, it is known that the Ministry of Food and Drug Safety recently instructed pharmaceutical companies to implement safety measures by setting the daily intake of Sitagliptin to 37ng. The original Januvia has already been fully prepared to meet the reinforced intake allowance. However, generic companies that have just entered the Sitagliptin market have a relatively short preparation time. It is expected that it will be a difficult fight for generic companies as it is to manage impurities within 30% of the current standard.
- Company
- 'Paxlovid reduces the risk of deaths in high-risk groups'
- by Jung, Sae-Im Jul 25, 2023 05:45am
- ‘Paxlovid,’ the oral COVID-19 treatment used to prevent progression to severe COVID-19, has finally removed its ‘temporary’ approval tag, 1 year and 7 months after its introduction to Korea. Although the drug has now been officially approved as a new drug, it is still not being well utilized on-site. Therefore, whether the misunderstandings and misconceptions regarding the drug can be cleared and be actively prescribed to patients by HCPs is gaining attention. The Ministry of Food and Drug Safety officially approved Pfizer’s COVID-19 treatment ‘Paxlovid (nirmatrelvir ritonavir tablets)’ on the 14th. The approval comes 1 year and 7 months after the company had received the emergency use approval (EUA) in December 2021. Paxlovid’s official approval was based on data from the Phase 2/3 EPIC-HR and EPIC-SR studies. The EPIC-HR study enrolled unvaccinated, non-hospitalized adults, aged 18 years and older, with confirmed COVID-19 who were at increased risk of progressing to severe disease. Results showed an 86% reduction in risk of COVID-19-related hospitalization or death from any cause through Day 28 in patients who initiated treatment with Paxlovid within 5 days of symptoms onset, compared to placebo. Recent real-world studies further support Paxlovid’s effect. According to a real-world study, the relative risk reduction effect of Paxlovid was confirmed in all high-risk patients regardless of whether they had been vaccinated or not. Based on this, the U.S. Food and Drug Administration (FDA) estimated in March that taking Paxlovid ‘could lead to 1,500 lives saved and 13,000 hospitalizations averted each week” in the U.S.’ Despite such supporting data, Paxlovid is not well used in Korea. The government and the company had carried out extensive promotional and education activities, but the prescription rate of Paxlovid in elderly patients remains in the 30% range, unable to break the 40% wall. Pfizer Korea Eun-Ji Kim, (COVID-19 Marketing Lead), and Hyemin Oh (Policy & Public Affairs Lead), Jae-Yoon Ryu, (Medical Sr. Manager), and Hyemin Oh (Policy & Public Affairs Lead) (Source: Pfizer Korea) On the 24th, Pfizer Korea held a meeting to celebrate the official approval of Paxlovid at its headquarters in Jung-gu, Seoul. Jae-Yoon Ryu, (Medical Sr. Manager) Eun-Ji Kim, (COVID-19 Marketing Lead), and Hyemin Oh (Policy & Public Affairs Lead) attended the event to correct the misunderstandings and explain the truth about Paxlovid. At the same time, the members shared the company’s plans on transitioning Paxlovid into a general medical system. The following are the common misunderstandings and truths about Paxlovid that were addressed at the event. # Misunderstanding 1: Paxlovid can only be used on patients with severe symptoms = Paxlovid’s is indicated for ‘the treatment of patients with mild-to-moderate COVID-19 whose condition is at high risk of progressing to severe disease, including hospitalization or death.’ However, in the field, there remains a strong impression that Paxlovid should only be used on patients whose conditions are so severe that they should be transferred to general hospitals immediately. And symptomatic therapy is still mainly used on high-risk patients with mild symptoms. This misconception is the biggest hurdle to increasing the prescription rate of Paxlovid in the high-risk group. To be clear, Paxlovid is not a symptomatic treatment- the drug was approved based on its evidence in preventing severe disease, in lowering the risk of serious illness and death in high-risk patients. Although the symptoms are getting milder with the mutation of the COVID-19 virus, the disease burden is still high and about 10 people still die from worsening COVID-19 every day. Even if the symptoms are mild, if you have any of the high-risk factors, you need to be prescribed Paxlovid. # Misunderstanding 2: Elderly patients that take many medications cannot use Paxlovid due to contraindications = That is one reason why patients find it difficult to receive Paxlovid prescriptions - Paxloivd has too many contraindications. Doctors avoid prescribing Paxlovid if a patient says they take other medications. 26 drug ingredients are known to be dangerous or are not allowed to be used in combination with Paxlovid. However, just the fact that you are using one of the 26 ingredients does not forbid your use of Paxlovid. Those who use 19 of the 26 ingredients can use Paxlovid if they discontinue use of their respective drugs or receive alternative medications. Paxlovid’s use is contraindicated only for the other 7 ingredients. # Misunderstanding 3: The drug is difficult to use as it is cumbersome to check all the contraindicated drugs = In Korea, HCPs can use the Drug Utilization Review (DUR) program to check for contraindications of each ingredient. If the doctor wants to use Paxlovid, Korea has a system in place that allows doctors to quickly check for contraindications. Of course, some ingredients like St. John’s Wort that are nutritional supplements are not listed in the DUR and have to be checked separately. However, most of the prescription drug ingredients can be checked automatically with DUR, and we have been distributing medication guides for pharmacists, educational material, and use guides for the elderly. We are also contemplating ways to increase patient convenience. For example, if patients need to be prescribed alternative medications, they have to pick through all of the medications they take to replace the ones in need. And they need to visit the pharmacy to do this. So we are trying to find ways to make it easier for patients to take alternate medications safely. # Misunderstanding 4: Paxlovid’s emergency use status will be converted and be subject to the general medical system in the first half of next year =Currently, the Korean government purchases Paxlovid in advance and supplies it free of charge to patients in need. We are discussing when this system should be converted into a general medical system. Please understand that there are still a lot of government purchases left, and we need to undergo the reimbursement process, therefore, it is not clear how its use will be converted to the private sector and whether there will be an overlapping period. However, the government and the company are continuing close discussions so that patients who need the drug do not experience inconvenience in the middle. It is not ordinary for a drug that is approved just now to be registered in the first half of next year. We will be going through all the procedures that existing new drugs go through, but are negotiating on the timeline for quicker listing. The company is doing its best to submit the data required for listing to the government in a timely manner.
- Company
- Cinqair’s quicker reimb renders reimb difficult for others
- by Eo, Yun-Ho Jul 25, 2023 05:45am
- Attention is rising on the progress of the two new asthma drugs that chose to receive reimbursement through the RSA track, unlike Cinquair. According to industry sources, GSK Korea’s ‘Nucala (mepolizumab)’ has recently been reviewed by the Health Insurance Review and Assessment Service’s Risk Sharing Agreement (RSA) subcommittee for reimbursement. However, no news has been heard for AstraZeneca Korea’s Fasenra (benralizumab)’ yet. Unlike Teva-Handok’s Cinqair (reslizumab), which opted to take the general reimbursement listing track, the two drugs faced difficulties after passing the Drug Reimbursement Evaluation Committee review in July. If Cinqair completes drug pricing negotiations and is listed with reimbursement, it becomes virtually impossible for the other two drugs to receive reimbursement through the RSA track. Although the RSA subcommittee reviewed Nucala’s reimbursement, whether it can continue on the reimbursement discussions for RSA also remains to be seen. No other drug has been reimbursed for severe asthma since the reimbursement approval of Novartis Korea’s ‘Xolair (omalizumab)’ in 2020 Although the three drugs seem similar to Xolair as all are indicated to treat ‘asthma,’ Xolair can only be used for allergic asthma. Therefore, the drugs’ indications do differ. However, the government used Xolair as a comparator for reimbursement review, and the drug price set using Xolair was too low for the three new biological agents to accept. This is why the companies had foregone the reimbursement listing process. Nucala’s efficacy was demonstrated through the Phase III DREAM, MENSA, and SIRIUS studies. Among these, MENSA is the main trial for the drug, and its results were published in the NEJM in 2014. The MENSA study was conducted on patients with severe asthma who experienced exacerbations despite the use of various controller medications including high-dose inhaled corticosteroids (ICS). In particular, patients whose blood eosinophil count was ≥150 cells/μL at screening (≥300 cells/μL in the previous year) enrolled in the study. The patients received mepolizumab or placebo and were assessed for the annualized rate of clinically significant exacerbations. Results showed that at Week 32, the rate of exacerbations was reduced by 47% among patients receiving intravenous mepolizumab 75mg and by 53% among those receiving subcutaneous mepolizumab 100mg, as compared with those receiving placebo. At week 32, patients receiving Nucala had also shown significant improvement in quality of life and showed higher levels of satisfaction in terms of asthma control.
- Company
- Original Forxiga’s sales strong despite generic entry
- by Kim, Jin-Gu Jul 24, 2023 05:26am
- Pic of Forxiga (left)·Xigduo Three months have passed since generic versions of the SGLT-2 inhibitor class diabetes treatment ‘Forxiga (dapaglifloin)’ were released en masse in Korea. Despite the release, the original product had successfully defended its lead, increasing outpatient prescriptions. The generic companies raised a combined prescription performance of KRW 5.9 billion in the first quarter after their release, increasing their influence to 18% of the total market. However, as more than 60 companies that have entered the competition, the average prescription performance per company was less than KRW 100 million. Despite entry of a large number of generics...Prescriptions of Forxiga, Xigduo rise 12% within the year On the 22nd, according to the market research institution UBIST, the outpatient prescriptions of single agent and combination dapagliflozin drugs in Q2 was KRW 32.2 billion. Among those, Forxiga and Xigduo posted combined sales of KRW 26.3 billion. Compared to the KRW 23.4 billion in Q2 last year, the sales of the two drugs rose 12% YoY. Generic drugs entered in bulk after Foxiga’s substance patent expired in April. Over 60 companies have released Xigduo generics in Q2 alone. The generic companies posted combined prescriptions of KRW 5.9 billion in Q2. Its share in the overall market increased to 18% in 3 months. Despite the increasing performance of generic drugs in the market, prescriptions of Forxiga and Xigduo have increased. Industry analysis that AstraZeneca is defending its performance by carrying out active promotion activities with its copromotion partner, Daewoong Pharmaceutical. Forxiga and Xigduo’s drug prices were not reduced despite the release of generics. Originally, the drug prices of Forxiga and Xigduo were scheduled to be reduced by 30% ex offico by the Ministry of Health and Welfare following the reimbursement listing of its same ingredient generics. However, AstraZeneca filed an administrative suit claiming that the disposition was unfair and requested that the disposition be delayed until the ruling is complete. The court cited the request for suspension of execution, and the drug prices of Forxiga and Xigduo had remained the same ever since. Combined prescription of generic drugs amount to KRW 5.9 billion...Average sales per generic company less than KRW 100 million As for the generic companies, each company produced less than KRW 100 million per company. So many companies have simultaneously released their generic versions that the prescriptions each company had made were at an insignificant level. In Q2, 62 companies had released their single-agent Forxiga generic drugs, raising KRW 3.9 billion in total. Each company made roughly KRW 62 million. Only 5 of the companies’ products - Boryung’s Trudapa, Hanmi Pharm’s Dapalon, Chong Kun Dang’s Exiglu, Aju Pharm’s Daparil, Dong-A ST’s Dapapro – made over KRW 200 million in Q2. 50 of the 62 companies (81%) that released their generic versions had made less than KRW 100 million in Q2. The situation was similar for the combination generic drugs as well. 32 companies had released their generic Xigduo versions and posted combined sales of KRW 2.1 billion. Each company made roughly KRW 65 million. Only 5 of the companies’ products - Hanmi Pharm’s Dapalon Duo, Boryung’s Trudapa M, Kyung Dong Pharma’s Dapamet SR, Daewon Pharmaceutical’s Dapawon-M, Aju Pharm’s Daparil Duo - made over KRW 200 million in Q2. 25 of the 32 companies (78%) that released their generic versions had made less than KRW 100 million in Q2.
- Company
- CDK4/6 anticancer drug is very active in early breast cancer
- by Jung, Sae-Im Jul 24, 2023 05:25am
- The activity of CDK4/6 inhibitors in early breast cancer is not limited to Verzenio. Kisqali also presented data on adjuvant therapy after successful surgery at ASCO held in June. Although they are the same class of drugs, the clinical designs of the two drugs in early breast cancer are different in many ways. First, Verzenio was targeted for high-risk patients with lymph node metastasis. On the other hand, Kisqali did not limit the presence of lymph node metastases. Relatively less risky patient groups were also included in the clinical trial. The dose and duration of administration are also different. Verzenio uses the same dose as for metastatic breast cancer, and after two years of administration, only endocrine therapy is continued. On the other hand, Kisqali lowered the dose by two-thirds and set the duration of administration to 3 years while conducting clinical trials. It appears to be intended to lower the risk of side effects by reducing the dose. CDK4/6 inhibitor options are increasing in early breast cancer, but their use in the field is still limited. This is because there are no drugs that have entered the right to benefit. HIRA reviewed Verzenio's early breast cancer reimbursement standards in May but did not set standards. There is also a prejudice against early cancer. It is a view that the patient group is wider than terminal cancer and that life is not in a relatively dangerous situation. At the same time, there are practical questions about whether it would be cost-effective to spend significant drug costs on all 100 people to reduce the risk of recurrence in several people. Dailypharm listened to changes and improvements in treatment strategies for early breast cancer through a conversation with Professor Nadia Harbeck, head of the breast center at Munich University, Germany, and Professor Lim Seok-ah, Department of Hematology and Oncology, Seoul National University Hospital. Professor Lim Seok-ah -There are increasing cases of CDK 4/6 inhibitors spreading to early breast cancer. How to expect to meet the prognosis and unmet needs of early breast cancer patients in terms of quality of life. =Professor Lim Seok-ah: Korea has a well-operated breast cancer screening system, so there are many early breast cancer cases. About 80% of breast cancers are of the HR+ type, and the number of patients with this type is relatively high among young people between the ages of 45 and 55. In the West, premenopausal female patients account for 20-30% of all breast cancer patients, but in Korea, they account for almost half. Young women's breast cancer has a slightly more aggressive characteristic, especially among the HR+ types, the luminal B type has a faster tumor growth rate than the luminal A type. In light of treatment experience, the proportion of patients with Luminal B type is higher in HR+ breast cancer among women in their 40s and 50s in Korea. In these patients, relapse is quite common during the first 2 to 3 years of endocrine therapy for 5 years after surgery. Personally, I believe that reducing the recurrence rate by using Verzenio for 2-3 years, when the recurrence frequency is high, can yield important results that increase the patient's chance and possibility of a full recovery. Korea has recently started to use ovarian function inhibitors and aromatase inhibitors as adjuvant therapy for HR+/HER2- breast cancer. Now, with the addition of Verzenio, we believe that the treatment strategy will proceed in the direction of improving the prognosis by reducing the recurrence rate of early breast cancer patients, including premenopausal women. =Professor Nadia Harbeck: Patients with HR+/HER2- early breast cancer had significant unmet needs in the past. Even if patients underwent endocrine therapy or chemotherapy, the data in terms of survival were not good. It is very encouraging that Verzenio can now be used in early patients to increase the chances of a full recovery. This is because cure is the ultimate treatment goal in early breast cancer. Professor Nadia Harbeck -Recently, following Verzenio, other CDK 4/6 inhibitors also released new data in the field of early breast cancer. What are the prospects for the use of CDK 4/6 inhibitors in the field of early breast cancer in the future? Along with this, there are opinions that administering drugs to 100 patients to prevent the risk of recurrence in about 3 patients is not cost-effective. What do you think? =Professor Lim: The appearance of CDK 4/6 inhibitors is providing hope to early breast cancer patients who have been fearful of recurrence due to limited treatment options. In addition, as clinical trials were conducted for patients with a high risk of recurrence, it plays an important role in preventing high-risk early breast cancer patients from spreading to recurrent and metastatic breast cancer. If other clinical studies are successfully completed, the use of CDK 4/6 inhibitors in reducing the risk of recurrence of early breast cancer will be broadened. Treatment for early breast cancer has a fixed duration. If the recurrence of a patient can be prevented through active therapeutic intervention in the early stage of breast cancer for a certain period of time, the social and economic medical costs of the patient in the stage of metastatic and advanced breast cancer can be reduced. When reviewing cost-effectiveness, these factors should also be considered. - Since Verzenio was approved in Germany last year, reimbursement was applied immediately. What are the criteria for determining the salary? What are the effects experienced in actual clinical settings? =Professor Harbeck: In Germany, reimbursement starts immediately for drugs approved by the European Medicines Agency (EMA). Verzenio's prescription and reimbursement are conducted according to the monarchE research standards, and overall, both medical staff and patients are having a fairly positive experience. As a participant in one of the actual monarchE clinical research sites, following the experience in the research process, prescriptions are being routinely conducted in the real world. In actual clinical settings, there are patients who need dose adjustment due to some diarrhea or fatigue at the beginning of treatment, but most of them are satisfied with the treatment and have excellent treatment compliance. Because patients know that they are at high risk of recurrence, they are very satisfied that there is a new treatment available to them that can protect them from the risk of recurrence.