- LOGIN
- MemberShip
- 2025-12-23 11:37:52
- Company
- Perjeta and Herceptin combination drug, Phesgo
- by Eo, Yun-Ho Aug 30, 2023 05:31am
- Phesgo, a subcutaneous injection-type combination of Perjeta and Herceptin, challenges insurance coverage registration. As a result of the coverage, Phesgo of Roche Korea is presented to the Cancer Disease Review Committee of the HIRA today (30th). This is the first case of an improved anti-cancer biobetter. Biobetter refers to drugs recognized by the Minister of the Ministry of Food and Drug Safety as having improved safety, effectiveness, and usefulness (medical compliance, convenience, etc.) compared to previously approved biological drugs, or as being progressive in pharmaceutical technology. In the case of Phesgo, by replacing Herceptin and Perjeta, which were used for intravenous injections, with fixed-dose subcutaneous injections, it was recognized for its innovativeness in improving patient convenience and reducing treatment time and was named as the first improved biologic drug for anticancer drugs. For example, if a patient with metastatic HER2-positive breast cancer who was receiving maintenance therapy every 3 weeks with Herceptin·Perjeta intravenous injection changes the therapy to Phesgo SC, the total time required for dosing and monitoring is 20 minutes from 270 minutes (90 minutes + 180 minutes). (5 minutes + 15 minutes), it is reduced by more than 90% compared to before. In addition, Pesco is a subcutaneous injection administered into the thigh rather than into a vein and can reduce blood vessels and nerve damage caused by repeated intravenous injection. In Korea, Phesgo was approved by the Ministry of Food and Drug Safety for the same indication as Perjeta, so it is expected that the subject of reimbursement will also be discussed according to Perjeta standards. The NCCN Guidelines state that Phesgo can replace Perjeta and Herceptin, and in fact, in the UK, 90% of patients treated with Perjeta and Herceptin changed their treatment to Phesgo one year after Pesco was launched, so Perjeta and Herceptin in Korea also A significant number of patients receiving treatment are expected to switch to Pesco. Meanwhile, Phesgo, a fixed-dose subcutaneous injection, confirmed non-inferior blood concentration and complete remission data compared to the intravenous Perjeta-Herceptin combination therapy in phase 3 clinical trial of FeDeriCa and confirmed a similar safety profile. Also, according to the phase 2 clinical PHranceSCa study on patient preference, 85% of patients preferred subcutaneous treatment to intravenous administration because of a shorter stay in the hospital and more convenient treatment administration. In addition, 87% of the patients who participated in the study responded that they would proceed with Pesco for the remaining breast cancer treatment.
- Company
- Keytruda posts sales of KRW 183.8 bil in 1H...unrivaled lead
- by Chon, Seung-Hyun Aug 29, 2023 05:28am
- The immuno-oncology drug Keytruda’s sales have been skyrocketing in the domestic drug market. In the first half of the year alone, the drug posted sales of over KRW 200 billion and secured its unrivaled lead in the market. The drug’s sales have been rising more steeply after the drug’s reimbursement was extended as first-line therapy last year. According to the market research institution IQVIA on the 24th, MSD Korea’s Keytruda posted the most sales, recording KRW 183.8 billion in 1H last year. With a 94.7% increase from the KRW 94.4 billion it had posted last year, the drug maintained its lead in the market. Keytruda posted sales of KRW 88.8 billion in Q1, which was a 117.1% YoY increase, followed by KRW 96 billion in Q2, up 77.9% YoY. Keytruda is an immune checkpoint inhibitor that inhibits PD-1 (programmed death 1) proteins expressed at the surface of activated T cells, thereby inhibiting its binding to PD-L1 and activates the immune system to treat cancer. The drug is currently approved for 16 cancers: ▲Lung cancer, ▲head and neck cancer, ▲ Hodgkin lymphoma, ▲urothelial carcinoma (bladder cancer), ▲esophageal cancer, ▲ melanoma, ▲renal cell cancer (kidney cancer), ▲endometrial cancer, ▲stomach cancer, ▲small intestine cancer, ▲ovarian cancer, ▲pancreatic cancer, ▲biliary tract cancer, ▲colorectal cancer ▲triple negative breast cancer, and ▲cervical cancer. It is indicated for the largest number of cancer types among cancer immunotherapies approved in Korea. It has shown accelerated performance since its reimbursement was extended to the first line last year. In March of last year, Keytruda’s reimbursement was extended to cover first-line treatment for non-small cell lung cancer. Keytruda sales increased 33.4% from 40.4 billion won in Q1 last year to KRW 53.9 billion in Q2. In Q3 and Q4 last year, it continued to show high growth, recording KRW 67.2 billion and KRW 78 billion, respectively. Keytruda's insurance ceiling price was lowered by 25.6% as the benefits range expanded. Considering the growth rate of 77.9% in the second quarter of this year, it is calculated that the amount of use has more than doubled in one year since the first treatment benefit was applied. Keytruda’s prescriptions have increased significantly after the reimbursement extension. Keytruda’s insurance ceiling price was reduced by 25.6% in March last year with its reimbursement extension. When considering its growth of 77.9% in Q2 this year, the calculation shows that its amount of use has more than doubled in one year since it started being reimbursed as a first-line therapy. Keytruda has maintained its lead position for 14 consecutive quarters since taking first place in Q1 2020. It has positioned itself as an unrivaled leader in the market, posting sales that are over 2.5 times larger than that of Lipitor, which is in second place. Keytruda’s sales are expected to exceed KRW 100 billion in quarterly sales within the year for the first time among drugs sold in Korea. Also, new drugs that were recently introduced by multinational pharmaceutical companies continued to show strong performance. Amgen's Prolia recorded sales of KRW 73.3 billion in 1H, up 32.1% from the previous year, and ranked third place. Prolia’s sales increased by 41.6% YoY to KRW 35.5 billion in Q1 and by 24.3% to KRW 37.9 billion in Q2. In Q2, Prolia had overtaken Lipitor’s rank for the first time and had risen to second place. Prolia is a biological osteoporosis treatment that targets the RANKL protein essential for the formation, activation, and survival of osteoclasts that destroy the bone. Its sales started to rise after it was applied reimbursement as a second-line treatment in 2017. After additionally being approved for reimbursement in the first line from April 2019, Prolia’s sales rose explosively. Last year, in 6 years since it was introduced to Korea, its annual sales exceeded KRW 100 billion for the first time. Prolia is copromoted by Chong Kun Dang in Korea. Sales of Ono Pharmaceutical’s cancer immunotherapy Opdivo increased 35.3% YoY to record KRW 69.4 billion in 1H year. Its sales had risen by 35.4% and 35.2%, respectively, in Q1 and Q2. Opdivo, which was approved in 2015, posted sales in the KRW 10 billion range until Q2 2021, then exceeded KRW 20 billion in Q3 2021, and exceeded KRW 30 billion in Q4 last year. The drug had recorded a high growth rate of 64.7% in 2 years from the KRW 66.7 billion it had earned in 2020 and posted annual sales that exceeded KRW 100 billion for the first time last year. Sales of Sanofi’s atopic dermatitis treatment Dupixent rose 33.7% YoY to record KRW 65 billion in 1H. Dupixent is the first targeted biologic for the treatment of moderate-to-severe atopic dermatitis that is not well controlled with topical therapies or who cannot use topical therapies. Sales of Dupixent, which was approved in March 2018, increased rapidly after it was approved for reimbursement for severe atopic dermatitis in January 2020. Among domestically developed new drugs, HK Inno.N’s gastroesophageal reflux disease treatment K-CAB’s sales had risen 15.0% YoY in 1H to record KRW 59.3 billion, and rose to 6th place. K-CAB, was released in March 2019. It has a new mechanism of action that inhibits gastric acid secretion by competitively binding to the proton pump and potassium ion located in the final stage of acid secretion.
- Company
- The synergy between technology and sales force
- by Chon, Seung-Hyun Aug 29, 2023 05:28am
- Domestically developed biosimilar products are prominent in the large anticancer drug Asastin market. Samsungbioepis' Onbevzi exceeded 10 billion won in quarterly sales in the first two years of its release, showing a 35% share. It is said that Boryung, which was the first biosimilar to enter the market and has differentiated strengths in anticancer drug sales, added to sales and maximized synergy. According to IQVIA, a pharmaceutical research institute on the 29th, the market size of Bevacizumab in the first half of last year was 59.9 billion won, up 36.1% from the same period last year. Sales in the first quarter were 29.5 billion won, up 39.8% from the previous year, and continued to grow at 30.4 billion won in the second quarter, a high growth rate of 32.8%. Bevacizumab is the original medicine of Roche's Avastin. It is an anticancer drug used for metamorphic direct colorectal cancer and metamorphic breast cancer, non-small cell lung cancer, advanced or metamorphic renal cell carcinoma, glioblastoma, epithelial ovarian cancer, topetic tube cancer, primary peritoneal cancer, and cervical cancer. Domestically developed biosimilars recently led the expansion of the Bevacizumab market. In the Avastin market, Samsung Bioepis released the Biosimilar in September 2021, with an additional entry from Celltrion and Alvogen Korea. Onbevzi's sales in the first half of the year were 20.3 billion won, up 246.9% from the previous year. Onbevzi's sales in the first quarter were 9.8 billion won, more than five times from 1.8 billion won in the same period last year, and recorded 10.5 billion won in the second quarter, up 157.0% from the previous year. OnBev is the first time that a biosimilar product released by Samsung Bioepis has exceeded 10 billion won in quarterly sales. Celltrion and Alvogen Korea, which entered the market this year, have quarterly sales of 100 to 200 million won. Onbevzi was the first to enter the market among biosimilar products and maximized the synergy by equipping it with a customized sales force. Samsung Bioepis signed a domestic exclusive sales contract with Boryeong shortly after OnBevji's domestic license. Boryeong is one of the domestic companies that has strengths in the area of anticancer drugs. Boryung established the anti-cancer division in May 2020. The organization, which was under the specialty medicine sector, was independent as a separate division. We have secured the rights to various anticancer drugs and biosimilars owned by domestic and foreign companies, and equipped with gems and notifications as a LBA strategy that buys the rights to the original anticancer drugs. Boryung also secured domestic rights for Samsung Bioepis' Avastin and Herceptin biosimilar products in 2021. Boryung is on the rise in the anticancer drug business in the first half of this year alone, with anticancer drug sales of 106.1 billion won, up 48% from the previous year. From Boryung's point of view, it is also enjoying the effect of improving performance while equipping high-market products. Samsung Bioepis Samphenet's sales in the first half of the year were 4.1 billion won, up 65.1% from the previous year. Although the sales volume is not large, the effect of Boryeong's sales force was noticeable. Sales of the original drug Avastin have not changed much. In the first half of last year, Asastin's sales were 38.9 billion won, up 2.9% from the previous year. Asastin's first- and second-quarter sales rose 0.4% and 3.6% year-on-year, respectively. Avastin showed a stable growth flow of 28.7 billion won, 30.2 billion won and 30.8 billion won from the first quarter to the third quarter of 2021, but it was 22 billion won in the fourth quarter, down 28.6% from the previous quarter. A decline in sales was inevitable due to the weakening of drug prices following the emergence of biosimilars. Samsung Bioepis was approved for Avastin's first biosimilar OnBevji in March 2021 and has been listed on the health insurance benefit list since September of the same year. In October 2021, the upper limit of 0.1g/4mL of Avastin was reduced by 30% from 33,387 won to 231,271 won due to the listing of OnBevji. Avastin 0.4g/16mL fell 30%. In principle, when biosimilars appear in the domestic drug price system, the upper limit of original medicines is 30% lower than before the patent expired. 'Innovative pharmaceutical companies, equivalent companies, domestic pharmaceutical companies, and foreign companies that have signed a joint contract, or items that Korea is the first licensed country or products produced in Korea' are guaranteed up to 80% of the original products before the patent expiration of both the original pharmaceuticals and biosimilars. Because Samsung Bioepis is not an innovative pharmaceutical company, the price of Avastin's drug has fallen to the previous 70% level. Avastin has experienced a decline in sales of the drug price reduction rate but has since formed similar-scale sales, minimizing the sales gap due to biosimilar penetration. Onbevji had a 34.5% share of the Bevacizumab market in the second quarter. Considering that there has been no additional drop in sales since the price drop by Asastin, Onbebge has actually created a new market worth 10 billion won in quarterly sales. As a result, it is analyzed that the drug price of the original drug also fell by 30% due to the entry of Onbevji, resulting in a significant effect on health insurance finances and the reduction of drug prices for patients.
- Company
- Verquvo busy securing prescriptions in KOR with reimb
- by Eo, Yun-Ho Aug 29, 2023 05:28am
- The new heart failure drug ‘Verquvo’ is busy securing prescriptions in Korea with its reimbursement listing. According to industry sources, Bayer Korea’s soluble Guanylate Cyclase (sGC) stimulator Verquvo (Vericiguat)’ passed the drug committees of Korea Anam Hospital, Chung-Ang University Gwangmyeong Hospital, Seoul National University Bundang Hospital, Samsung Medical Center, Pusan National University Hospital at Yangsan, Yeosu Jeil Hospital, Ewha Womans University Mokdong Hospital, Chonnam National University Hospital, etc. Bayer is currently undergoing review processes at other major general hospitals and plans to make Verquvo’s prescription available at most hospitals nationwide within the year. Verquvo, which is a chronic heart failure treatment, passed the Drug Review Evaluation Committee of the Health Insurance Review and Assessment Service in May after being approved in November 2021, and recently completed drug pricing negotiations with the National Health Insurance Service and is scheduled to be listed next month in September. Since the drug is a new option introduced long after the introduction of Novartis’s ‘Entresto (valsartan/sacubitril, it is expected that the drug will land quickly in the field after reimbursement. As a result, attention is also focused on how Korea’s heart failure treatment market currently occupied by Entresto and antidiabetic SGLT-2 inhibitors will change with the introduction of Verquvo, which has a new mechanism of action. Verquvo can be prescribed as a combination therapy to reduce the risk of cardiovascular death and heart failure hospitalization following hospitalization for heart failure or in need of outpatient intravenous (IV) diuretics in adults with symptomatic chronic heart failure and an ejection fraction less than 45%. The efficacy of the drug was demonstrated through the Phase III VICTORIA trial. The trial enrolled 5,050 adult patients with symptomatic chronic heart failure (New York Heart Association [NYHA] class II-IV) and left ventricular ejection fraction (LVEF) of less than 45% following a worsening heart failure event. A worsening heart failure event was defined as heart failure hospitalization or the use of outpatient IV diuretics for heart failure prior to randomization. 59.7% of the participants had been receiving 3-drug combination therapy, and 41% were severe patients - NYHA Class III or NYHA Class IV. In the trial, patients received up to the target maintenance dose of Verquovo 10 mg or a matching placebo combination with another heart failure therapy. Results showed that at a median of 10.8 months of follow-up, the risk of death from cardiovascular disease or first hospitalization due to heart failure was about 10% lower than that of the placebo group, and the trial met its primary efficacy endpoint with an annual absolute risk reduction of 4.2%.
- Company
- Price negotiation period for Spinraza and Evrysdi extended
- by Eo, Yun-Ho Aug 28, 2023 05:21am
- The companies of the spinal muscular atrophy (SMA) treatments ‘Spinraza’ and ‘Evrysdi’ failed to conclude drug pricing negotiations for their drugs within the deadline and began extended negotiations. As a result, Spinraza reimbursement extension and Evrysdi’s new listing will only be possible in October at the earliest. According to industry sources, the reason for the delay in negotiations for the two drugs is known to be the differences in opinions between the companies and the authorities on the Expenditure Cap that should be set, which is a type of Risk Sharing Agreement (RSA) scheme that is applied to drugs eligible for reimbursement through the pharmacoeconomic evaluation exemption system. In other words, the companies were unable to agree on the cap that should be applied, in consideration of factors such as the expected amount of use and the number of patients. Discussions on reimbursement and reimbursement extension of SMA treatments had seemingly gained speed in line with the movement that pressed for the abolition of Spinraza’s reimbursement suspension standard, but discussions were prolonged after a certain group requested a wider coverage than what the government and pharmaceutical companies expected. At the time, the government announced plans to proceed with discussions on Evrysdi’s listing after agreeing on the reimbursement extension standards for Spinraza. Both drugs were finally put on the final table for negotiations, but the news on the conclusion of the negotiations can only be expected in September. Meanwhile, SMA treatments currently reimbursed in Korea include Spinraza and Novartis Korea's one-shot treatment, 'Zolgensma (Onasemnogene abeparvovec-xioi))'. Spinraza is administered directly into the spinal fluid, and Zolgensma is administered intravenously. Evrysdi, which is attempting new listing, is an oral drug that reduces the social and economic burden patients and their caregivers face due to injections, such as school/work interruption, transportation costs, and nursing care It also has the advantage of being able to reduce drug costs for infant patients as it offers customized prescriptions by age and weight.
- Company
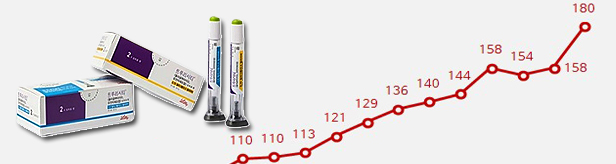
- Trulicity's share reaches 99.8%
- by Kim, Jin-Gu Aug 28, 2023 05:21am
- Trulicity Trulicity, a GLP-1 analog class diabetes treatment, once again set the record for highest sales. However, it is unclear whether this upward trend will continue in the second half. This is because it is said that some disruptions have occurred in domestic supply due to a shortage of global distribution supplies. Ozempic and Rybelsus, which are considered strong opponents, are waiting for release. In the pharmaceutical industry, if the two drugs are added in earnest, it is expected that the diabetes treatment market, which is dominated by Trulicity, will fluctuate greatly. According to IQVIA, a pharmaceutical market research institute, on the 26th, Trulicity's sales in the second quarter were 18 billion won. Compared to the second quarter of last year, it increased by 24.8% in one year. Trulicity is a GLP-1 analog class diabetes treatment. GLP-1 analogs are drugs developed using the GLP-1 hormone involved in blood sugar control in the body. The GLP-1 hormone promotes insulin secretion right after a meal to drop blood sugar, and when blood sugar drops below a certain level, it reduces insulin secretion to help prevent hypoglycemia. It has been 7 years since it was released in Korea, but it is still growing rapidly. Trulicity exceeded 10 billion won in quarterly sales in the third quarter of 2019 and exceeded 15 billion won in sales in the second quarter of last year. After that, it stagnated in the fourth quarter of last year and the first quarter of this year, but set a new record again in the second quarter. Even though it was released as the latest among the drugs in the same class, it won the competition with Victoza, Lyxumia, and Byetta, and in 2017, it virtually solidified its solo system. As of the second quarter of this year, Trulicity's market share reached 99.8%. Shortage of domestic supply and notice of the emergence of competitive drugs. In June, Eli Lilly notified its partner Boryeong that Trulicity would be difficult to supply in Korea. It is said that the clinical site has been struggling with the supply of Trulicity since last July. In this regard, Eli Lilly's position is that there is a disruption in supply due to the increase in global demand. On the other hand, some in the pharmaceutical industry argue that Trulicity's production has decreased relatively as Eli Lilly focused on producing Mounjaro, a next-generation drug, at the headquarters level. The timing of the release of competing drugs is also considered a variable. In the pharmaceutical industry, Novo Nordisk's Ozempic and Rybelsus are considered strong competitors to Trulicity. Ozempic is a long-acting, once-a-week injection of the same type as Trulicity, and Rybelsus is an oral drug. Rybelsus is taken once daily. Novo Nordisk received licenses for Ozempic in April and Rybelsus in May, respectively. It is said that Ozempic has already overtaken Trulicity in the global market. However, the official launch of Ozempic in Korea has been temporarily suspended due to a lack of global supply.
- Company
- Discussion on expanding coverage for Leclaza begins
- by Eo, Yun-Ho Aug 28, 2023 05:21am
- Attention is focused on expanding insurance coverage for the first-line treatment of the anticancer drug Leclaza. According to related industries, Yuhan Corporation's 3rd generation EGFR TKI Leclaza will be scheduled for the HIRA on the 30th. The specific indication is 'primary treatment for EGFR active non-small cell lung cancer'. Leclaza obtained approval from the Ministry of Food and Drug Safety for the indication in June. If it is presented to the review committee, the evaluation discussion will proceed within two months of approval. This drug passed the review committee about a month after January 2021, when it was first approved as a second-line treatment, and was listed in July of the same year. As a domestic new drug, it is showing rapid speed. Considering that it usually takes 4 to 5 months for an anti-cancer drug to be submitted to the cancer screening after applying for benefits, this is a considerable speed. Leclaza confirmed a statistically significant improvement in progression-free survival in first-line non-small cell lung cancer therapy through LASER301 in a phase 3 study. As a result of the analysis of the primary endpoint PFS in this study, the Lazertinib treatment group improved PFS statistically with 20.6 months in the Leclaza administration group and 9.7 months in the control Iressa administration group. Yuhan Corporation is operating an Early Access Program that provides free drugs to patients until they can receive health insurance benefits. The program will run from the date of each IRB approval until the time of Lazertinib's reimbursement standard expansion. In Korea, EGFR TKIs such as AstraZeneca's Iressa and Roche's Tarceva, second-generation drugs Giotrif and Vizimpro, and AstraZeneca's Tagrisso, like Leclaza, are currently prescribed in Korea.
- Company
- New drug Shingrix leads shingles vaccine mkt
- by Chon, Seung-Hyun Aug 28, 2023 05:21am
- GlaxoSmithKline's shingles vaccine 'Shingrix' took the lead in Korea’s market just 7 months after its release. With its powerful shingles prevention effect, its sales surpassed that of its competitors. SkYZoster, a homegrown vaccine, had taken the lead in the market for the first time at the end of last year but was overtaken by Shingrix in just 2 quarters. According to the market research institution IQVIA on the 26th, the market for preventive vaccines for shingles was KRW 45.4 billion in 1H last year, which was a 123.3% increase from the KRW 20.4 billion in 1H last year. The shingles vaccine market grew 147.0% YoY to record KRW 21.9 billion in Q1 this year, then again rose 124.1% to KRW 23.5 billion. The shingles vaccine market, which had only been KRW 10.5 billion in Q4 last year, saw a more than twofold increase in a single quarter. This abrupt market expansion is related to the introduction of the new vaccine Shingrix. Shingrix, which started being vaccinated in December last year, showed off its presence by occupying 28.9% of the market with sales of 6 billion won in Q1 this year. In Q2, the drug quickly rose to lead the shingles vaccine market with sales of KRW 11.1 billion. Shingrix's share of the shingles vaccine market in Q2 reached 47.4%. In other words, within 7 months of its launch, the vaccine has occupied about half of the entire market. The greatest benefit of Shignrix is its strong shingles prevention effect. In a Phase III clinical trial (ZOE-50) that was conducted on adults aged over 50 years of age, Shingrix showed a 97.2% efficacy compared to the non-vaccinated group at 3.2 years of follow-up, and in another Phase III clinical trial (ZOE-70) conducted on adults aged 70 years and above, Shingrix showed an 89.8% efficacy at 3.7 years of follow up. This is superior to the 5% protection in adults aged over 50 years of age and 41% in adults aged 70 years and above demonstrated with the use of Zostavax. The prevention rate of SKYZoster is also known to be similar to Zostavax. Also, Shingrix’s safety profile was confirmed through 5 clinical trials that were conducted on immunocompromised patients aged 18 years and older. Based on such evidence, patients who received autologous hematopoietic stem cell transplantation or those with solid cancer, blood cancer, or solid organ transplant patients who have an increased risk of shingles are also eligible to receive vaccination with Shingrix. At the time of its release, Shingrix's significantly higher price than existing vaccines was pointed to as an obstacle to the vaccine’s early settlement into the market. vaccines. The price of Shingrix, which is administered two times in total, is set at around KRW 500,000 to 600,000. This is more than twice as high as the existing vaccine, which costs KRW 150,000 to 200,000. However, Shingrix rapidly increased its market share in the market thanks to its superior efficacy despite the high price. Also, the sales support from 2 domestic pharmaceutical companies contributed to Shingrix’s rapid market penetration. GSK started domestic sales of Shingrix in partnership with two companies, GC Biopharma and Kwangdong Pharmaceutical. Although other existing shingles vaccines like SKYZoster and Zostavax also saw a sales increase from last year, their market share declined due to Shingrix. SK Bioscience’s SKYZoster’s sales had risen 83.2% YoY to record KRW 16.7 billion in 1H this year. SKYZoster led the shingles market in Q1 with sales of KRW 9.5 billion, which is a 151.5% YoY increase. SKYZoster’s sales continued to rise in Q2 to record KRW 6.5 billion, a 31.1% YoY increase, it lost its lead to Shingrix at the time. Sky Zoster is a live attenuated vaccine for the prevention of shingles that was developed by SK Bioscience with its proprietary technology. The vaccine has proven its non-inferiority compared to its competitor (Zostavax) in adults aged 50 or older in 8 domestic clinical institutions. In October 2017, SK Bioscience obtained approval for SKYZoster from the Ministry of Food and Drug Safety for 'preventing shingles in adults over the age of 50'. Before its approval, MSD's Zostavax was the only vaccine on the market. SKYZoster’s sales surpassed Zostavax for the first time since its release in Q4 year and took the lead. It maintained the lead for two consecutive quarters until Q1 this year. However, due to Shingrix's rapid rise, the vaccine lost its lead after 2 quarters. Zostavax’s sales in 1H this year was KRW 11.9 billion, up 2.6% YoY. Zostavax’s sales in Q1 this year rose by 18.9% YoY to reach KRW 6 billion but then fell 10.1% to KRW 5.8 billion in Q2. Zostavax lost its lead to SKYZoster in Q4 last year and then fell to third place with the introduction of Shingrix. Zostavax only occupied 24.8% of the market in Q2.
- Company
- Hanmi Pharm starts P1T on its next-gen immuno-oncology drug
- by Chon, Seung-Hyun Aug 25, 2023 06:06am
- On the 22nd, Hanmi Pharm announced that it has recently received approval to initiate a domestic Phase I trial for its next-generation immuno-oncology drug ‘‘BH3120(PD-L1/4-1BB BsAb)’ from the Ministry of Food and Drug Safety. In May, the company had previously received approval from the US FDA for the same drug candidate after submitting an Investigational New Drug Application. BH3120, which is be codeveloped by Hanmi Pharm and Beijing Hanmi Pharm, is a new drug candidate that applies the company’s, bispecific antibody platform technology ‘Pentambody’ that allows for one antibody to bind to two targets at once. The drug offers the benefit of providing both immunotherapy (that activates immune cells) and targeted therapy (that specifically targets cancer cells). BH3120 is an immunoglobulin G (IgG) like dual antibody designed that has biased binding affinities against PD-L1 and 4-1BB. The different binding affinities that were designed based on various studies are expected to induce better efficacy and safety. BH3120 has a dual antibody mechanism of action that targets both 4-1BB and the PD-L1 expressed on the surface of cancer cells that specifically elicit antitumor activities in the tumor microenvironment (TME), and has shown a potent antitumor effect by activating immune cells within cancerous tissues. In addition to BH3120’s benefits as monotherapy, Hanmi Pharm also confirmed the drug’s strong synergistic effect as combined therapy, as its use in combination with a PD-L1 inhibitor resulted in the disappearance of cancerous tissue. No toxicity or immune system-related side effects were observed with BH3120 in the safety study that was conducted on primates and was confirmed to have a superior safety profile compared to same-class competitors that are currently being developed. The company presented its study results at the American Association for Cancer Research (AACR) Annual meeting that was held in April this year. The results showed that a clear decoupling of immune activity between cancer and normal tissues was observed, signifying the possibility of the development of a new immuno-oncology drug that minimizes the side effects of existing immuno-oncology drugs Based on the preclinical study results, the company plans to start a global Phase I trial on BH3120 in the US and Korea within this year. An official from Hanmi Pharm said, “BH3120 is the first project that uses the next-generation bispecific antibody platform technology ‘Pentambody’ for a global clinical trial. We believe ‘Pentambody’ will follow Hanmi’s proprietary ‘Lapscovery’ technology and continue to create future value for the company.
- Company
- Company Roche and Drug Keytruda will lead industry in 5 yrs
- by Kim, Jin-Gu Aug 25, 2023 06:06am
- A projection has been made that Roche will become the top global-grossing pharmaceutical company 5 years from now in 2028. Evaluate, the global pharmaceutical market analysis institution that released such projections, also predicted that Keytruda would take over Humira’s throne and become the No. 1 grossing drug among pharmaceuticals. On the 24th, KoreaBIO cited Evaluate’s data and predicted the top-grossing pharmaceutical companies and drugs for 2023. According to KoreaBIo’s projections, biopharmaceutical companies that have been producing results will be in the top ranks this year. The top-grossing company 5 years from now will be Roche, supported by the sales of its immuno-oncology drug ‘Tecentriq,’ multiple sclerosis treatment ‘Ocrevus’ and macular degeneration treatment disease drug ‘Vabysmo.’ No.2 and No.3 projections were the U.S. companies Merck and AbbVie, respectively. However, the difference in revenue between the top 3 companies is expected to be small. No. 4 was expected to be Johnson & Johnson. Top 10 Grossing ETC Pharmaceutical Companies in 2028 Pfizer, which ranked No.1 with unrivaled sales last year was thought to fall to 5th place. In addition, Novartis, AstraZeneca, Sanofi, Novo Nordisk, and Eli Lilly were expected to make the top 10 sales list. Compared to the current status, Novo Nordisk and Eli Lilly are new to the top 10 list in 2028, taking the place of BMS and GSK. The expectation was that sales of diabetes and obesity drugs that the companies had recently released would surge, leading to a rise in the rankings. The immuno-oncology drug Keytruda is expected to take over the top spot in terms of global sales. In particular, it is predicted that Keytruda will rise to No. 1 this year, surpassing Humira, which had held the lead until now. In addition to Keytruda, immuno-oncology drugs such as BMS's Opdivo, Roche’s Tecentriq, and AstraZeneca's Imfinzi are also expected to make the Top 10. If the current upward trend continues, the total sales of immuno-oncology drugs are expected to surge from $40 billion in 2022 to $71 billion in 2028. In addition, Sanofi’s atopic dermatitis treatment ‘Dupixent,’ Eli Lilly’s antidiabetic treatment ‘Mounjaro,’ Johnson & Johnson’s multiple myeloma treatment ‘Darzalex,’ AbbVie’s autoimmune disorder treatment ‘Skyrizi,’ Gilead Science’s HIV treatment ‘Biktarvy,’ Merck’s cervical cancer vaccine ‘Gardasil,’ and Vertex Pharmaceutical’s ‘Trikafta’ are also expected to make the Top 10 in 2028. Top 10 Selling Drug Projections in 2028