- LOGIN
- MemberShip
- 2025-12-22 21:24:56
- Company
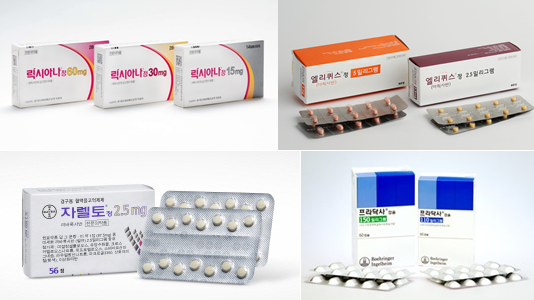
- 'Lixiana' dominates the DOAC market in KOR
- by Kim, Jin-Gu May 09, 2024 05:53am
- (clockwise from top-left) Lixiana, Eliquis, Pradaxa, and Xarelto. Daiichi Sankyo Korea’s 'Lixiana (edoxaban)' continues to dominate the market for Direct Oral Anti-Coagulant (DOAC). BMS’s 'Eliquis (apixaban)' sales have recently experienced a slowdown, and Bayer’s 'Xarelto (rivaroxaban)' prescription performance significantly dropped after the generic launch. The sales of Xarelto generic nearly chased the original product three years after its launch. The pharmaceutical industry eyes on the substance expiry of Eliquis this September. Eliquis generics that withdrew from the market after the Supreme Court’s ruling last 2021 may re-enter the market around the time of patent expiry, and a significant shift in the market is expected. Lixiana, 9% up in one year… Eliquis growth has been stalled According to medical market research firm UBIST, the market of DOAC treatment outpatient prescriptions in Q1 in South Korea amounted to KRW 62.9 billion, up 6% over a year compared to KRW 59.3 billion in Q1 last year. The mechanism of action of DOAC treatment is based on directly affecting the blood coagulation factor, thereby preventing blood clots. It is expanding its usage as it replaces warfarin, which prevents vitamin K metabolism. In South Korea, Xarelto was approved in 2009, followed by Pradaxa and Eliquis in 2011. Pradaxa and Eliquis were also approved in 2015. When the product was launched, it was called New Oral Anti-Coagulant (NOAC). Ten years after its launch, the term was changed to Direct Oral Anti-Coagulant (DOAC) to indicate that the product directly affects the coagulation factor. Four original products have seen a shift in the market. The top-selling product, Lixiana, is strengthening its position as the market leader. Prescription sales of Lixiana in Q1 of last year were KRW 27.7 billion, up 5% over a year compared to KRW 25.5 billion in Q1 of last year. Although it was launched late, its prescription performance grew fast, reaching the top after 2019. Last year, Lixiana became the first DOAC product to reach annual prescription sales of KRW 100 billion. Quarterly DOAC prescription performance (unit: KRW 100 million, source: UBIST). The second-ranked Eliquis has seen a stall in the growth recently. Eliquis prescription sales have seen a 2% growth, increasing from KRW 19 billion in Q1 of last year to KRW 19.3 billion in Q1 of this year. After Q3 of 2022, Eliquis prescription sales remain around KRW 19 billion. As Lixiana sales grew fast while Eliquis sales stalled, the sales gap between the two products is widening. The difference in the prescription sales between these two products in Q1 of this year amounted to KRW 8.5 billion. It continues to widen every year, with a difference of KRW 4.4 billion in Q1 of 2022 and KRW 6.5 billion in Q1 of 2023. Low Xarelto generics sales… Riroxban>Riroxia>Rivoxaban Over the last two years, Xarelto has experienced a significant decrease in prescription sales among the major DOAC products. Xarelto’s prescription sales in Q1 this year amounted to KRW 7.6 billion, down 5% compared to KRW 8 billion in Q1 last year. Its prescription sales declined by almost half over the past two years compared to KRW 14.8 billion in Q1 of 2022. Xarelto’s prescription sales decline is likely due to its patent expiration and generic release that followed. Xarelto generic was launched first in Q2 of 2021. Xarelto’s prescription sales decline is likely due to its patent expiration and generic release that followed. Xarelto generic was launched first in Q2 of 2021. In Q3 of 2022, the stoppage of reducing drug prices for Xarelto due to an administrative suit was lifted. When the pricing of Xarelto decreased, prescription sales started to decline. Eventually, the presence of Xarelto generics increased while the sales of the original prescription gradually decreased. Xarelto generics have gradually increased their prescription sales, nearly catching up with those of the original products. As of Q1, the net prescription sales of generic amounted to KRW 5.9 billion, up 61% over a year compared to KRW 3.7 billion in Q1 of last year. According to Q1 prescription performance report, Hanmi Pharm’s 'Riroxban' generated KRW 1.6 billion, CKD Pharm’s 'Riroxia' generated KRW 1.2 billion, and Samjin Pharmaceutical’s 'Rivoxaban' generated KRW 1.2 billion. Trends in prescription performance of rivaroxaban-containing original product and major generics (unit: KRW 100 million, source: UBIST). Pradaxa, another original DOAC product, is also facing a significant sales slowdown. In Q1, Pradaxa prescription sales amounted to KRW 2.4 billion, down 25% year over year. Eliquis’s substance patent set to expire this September…generic re-entry predicted The future variable in this market is the expiration of Eliquis' patent. Eliquis' substance patent expires on September 9th of this year. Generic manufacturers are waiting for the expiry of the substance patent, with Eliquis' formulation patent set to expire in 2031 after being invalidated. Generic companies won both the first and second trials related to substance patents in 2018 and 2019, respectively. After June 2019, Eliquis generic products were subsequently launched and have since been steadily growing in prescription sales. However, the circumstances changed when the Supreme Court ruled on the reversal of the original decision in April 2021. Generic companies have retrieved their products that could potentially infringe on the patent. On the contrary, Eliquis successfully regained its dominant position in the market for DOAC treatments containing the apixaban ingredient. With the substance patent set to expire this September, a flood of Eliquis generics is possible. Major generic manufacturers have experience releasing their products and are expected to expand their influence in the market quickly. On the other hand, it is expected that Eliquis will experience a sharp decline in prescription performance following the release of generics. From BMS's perspective, there is no justification for delaying the government's price reduction decision following the generic reimbursement listing. Due to this, the pharmaceutical industry anticipates that Xarelto will further strengthen its dominant position in the DOAC market.
- Company
- Novel targeted therapy for gastric cancer 'Vyloy' may launch
- by Eo, Yun-Ho May 09, 2024 05:52am
- 'Vyloy,' a targeted therapy for gastric cancer with a novel mechanism of action, is expected to be launched in South Korea. According to industry sources, Astellas Korea has submitted an application for approval of Vyloy (zolbetuximab), a treatment for claudin 18.2 (CLDN18.2) positive, HER2-negative, unresectable, advanced, or recurrent gastric cancer. In March, it acquired the final approval in Japan. Vyloy is a monoclonal antibody targeting CLDN18.2, which is expressed in the stomach. Its mechanism of action involves binding to CLDN18.2, a protein expressed on the cancer cell membrane of gastric epithelial cells. The efficacy of Vyloy was proven through the Phase 3 SPOTLIGHT and GLOW clinical trials. The SPOTLIGHT trial, which enrolled 557 adult patients with metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma, compared the efficacy and safety of zolbetuximab plus mFOLFOX6 combination therapy (fluorouracil, leucovorin, and oxaliplatin) to placebo plus mFOLFOX6. In the clinical trial, zolbetuximab combination therapy recorded progression-free survival (PFS), the primary endpoint, of 10.6 months, 8.7 months longer than the comparator. Additional clinical results showed that zolbetuximab combination therapy had an overall survival (OS) of 18.2 months, which was improved by 2.7 months compared to the comparator. In the GLOW study, zolbetuximab combination therapy improved PFS and OS. The study compared zolbetuximab plus CAPOX therapy (capecitabine and oxaliplatin) to placebo plus CAPOX therapy. The clinical results demonstrated that zolbetuximab combination therapy had a PFS of 8.2 months, 1.4 months longer than the comparator. The OS of zolbetuximab combination therapy was 14.4 months, compared to 12.2 months for the comparator. The common side effects related to safety were nausea and vomiting. Meanwhile, Vyloy is in progress for the discussion with the U.S. FDA, Europe, and China approvals. In the United States, its approval was rejected once over manufacturing issues.
- Company
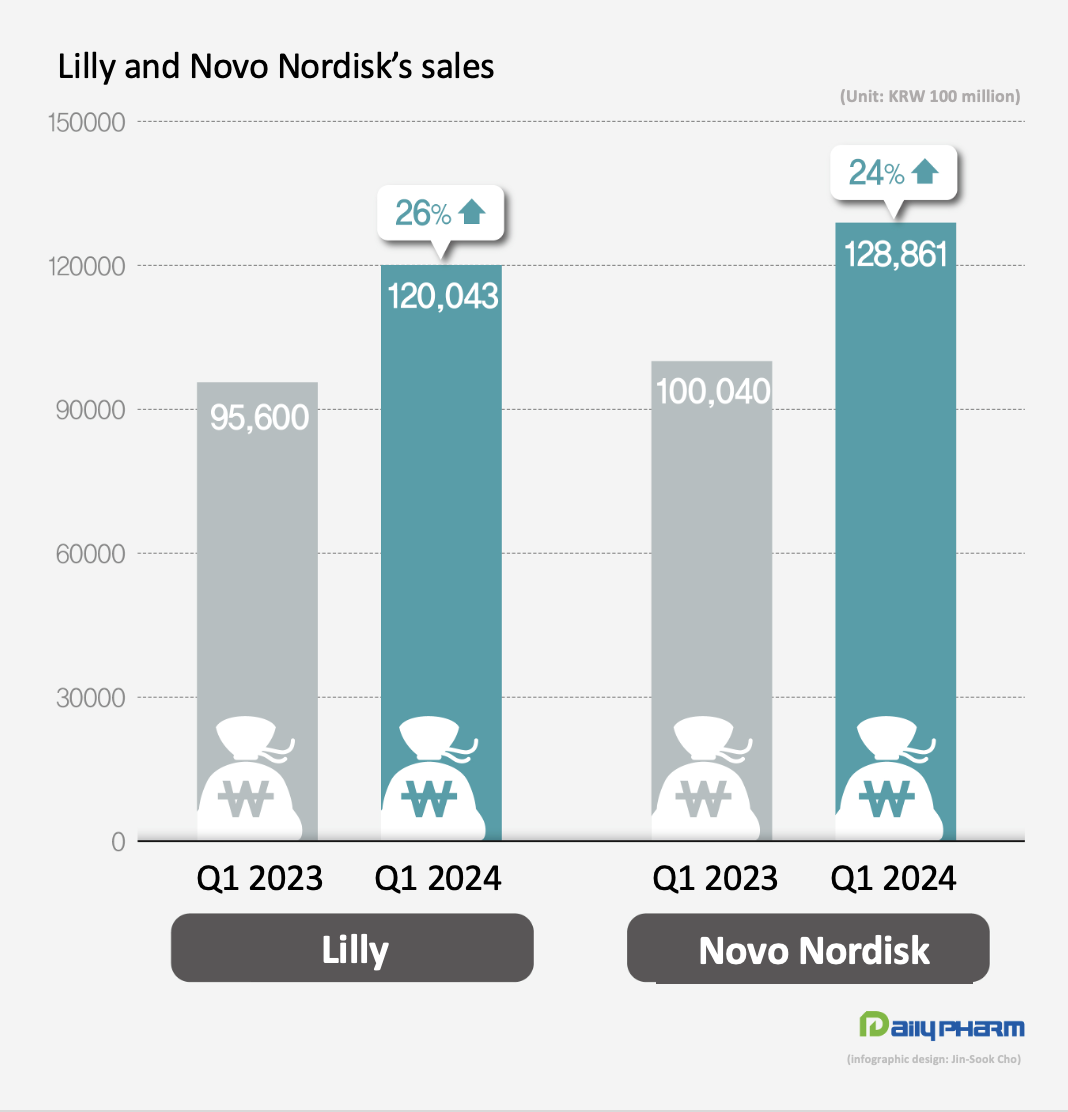
- Novo Nordisk and Lilly post strong performance
- by Son, Hyung-Min May 08, 2024 05:48am
- The performance of Novo Nordisk and Eli Lilly, which released blockbuster diabetes and obesity drugs, have improved greatly in Q1 this year. Novo Nordisk's GLP-1 class diabetes drugs together generated KRW 10 trillion in quarterly sales. Lilly's obesity drug Zepbound posted sales of KRW 700 billion in Q1 this year, reaching nearly 1 trillion won in only half a year since its launch. According to KoreaBio data on the 3rd, Novo Nordisk's first-quarter sales rose 24% YoY to KRW 12.886 trillion (DKK 65.349 billion). The exchange rate was based on the basic rate of exchange as of May 2. Novo Nordisk's diabetes medicines generated sales of KRW 9.8456 trillion, up 24% YoY. In particular, sales of GLP-1 diabetes medicines surged 32% YoY to KRW 6.8981 trillion. Novo Nordisk's GLP-1 class diabetes drugs include Ozempic, Rybelsus, and Victoza, which are semaglutide and liraglutide drugs. Sales of obesity treatments totaled at KRW 2.1759 trillion, up 42% from Q1 2022. Specifically, Wegovy’s sales rose 107% to KRW 1.849 trillion. As of February 2024, the global market share of Novo Nordisk's diabetes treatments was 34%, including 35% in North America, 30% in Europe and 32% in China. Its global share of obesity medicines amounts to 85%. Lilly sees strong sales of diabetes and obesity drugs Lilly also reported first-quarter sales of KRW 12.43 trillion (USD 8.77 billion), up 26% YoY, driven by new diabetes and obesity drugs. The increase was driven by the company’s new products. Sales of its new products launched in 2022 and thereafter jumped 495%, bringing in KRW 3.27 trillion in Q1, up from KRW 550 billion in Q1 last year. Mounjaro’s revenue increased 218% from KRW 780 billion in Q1 last year to KRW 2.48 trillion in Q1 this year, recording a revenue of KRW 2.08 trillion in the U.S. alone. Mounjaro is a type 2 diabetes treatment developed by Eli Lilly that has a dual effect on glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). By acting on GLP-1 and GIP, Mounjaro provides further blood glucose control. Mounjaro has also been shown to have dramatic weight loss benefits. Last year, Lilly also launched Zepbound, an obesity drug that contains the same substance as Mounjaro, after confirming its weight-loss benefits in clinical trials. The drug has become a global blockbuster only half a year after its launch, posting sales of KRW 71 billion in Q1. Demand for GLP-1 class drugs surge...company will address supply issue Novo Nordisk and Lilly are working to ensure smooth production of their GLP-1s, which are experiencing supply shortages. Currently, GLP-1 medicines are experiencing global stock-outs due to a surge in demand. Novo Nordisk plans to accelerate production by acquiring Catalent’s plant from Novo Holdings. Since Novo Holdings acquired Catalent last year, it has handed over 3 plants to its subsidiary, Novo Nordisk. Lilly has recently signed contract development and manufacturing organizations (CDMOs) with National Resilience in the U.S. and BSP Pharmaceuticals in Italy to increase its production capacity. To further increase its production facility in the mid-to long-term, Lilly recently acquired Nexus Pharmacutical's manufacturing facility in the U.S. and broke ground on a parenteral (injectable) manufacturing plant in Germany, both of which are expected to be operational by the end of 2025 and 2027, respectively.
- Company
- Prescriptions for cephalosporin antibiotics double in 3yrs
- by Chon, Seung-Hyun May 08, 2024 05:48am
- The popularity of antibiotics in the outpatient prescription market continues. The prescription market for cephalosporin-class antibiotics has nearly doubled in the past 3 years. This ‘heyday’ in the cephalosporin prescription market is continuing, fueled by the steady increase in flu and cold cases since the end of the pandemic. According to the market research institution UBIST, the outpatient prescription market for cephalosporin antibiotics was KRW 80.4 billion in Q1 last year, up 11.3% year-on-year. Compared to the KRW 64.1 billion it posted in Q1 2022, the market has expanded 24.4% in 2 years. Cephalosporin antibiotics, which are also called ‘Cepha antibiotics,’ are antibiotics used to manage a wide range of conditions including pneumonia, sore throat, tonsillitis, and bronchitis. The prescription market for cephalosporin antibiotics has undergone significant changes during the pandemic. The prescription market for cephalosporin antibiotics shrank by 29.3% from KRW 60.2 billion in Q1 2020 to KRW 42.6 billion in Q1 2021. In the early days of the COVID-19 pandemic, the prescription market for cephalosporin antibiotics shrank significantly, with the number of patients with infectious diseases such as flu and colds plummeting due to increased personal hygiene measures such as hand washing and mask-wearing. However, the demand for cephalosporin antibiotics surged with the explosive rise in the number of COVID-19 cases in late 2021. In Q1 2022, the prescription value of cephalosporin antibiotics began to rebound, reaching KRW 64.1 billion, up 50.6% YoY. Even after the end of the pandemic last year, the demand for cephalosporin antibiotics increased due to a steady number of confirmed daily COVID-19 cases and the rising number of flu and cold cases that arose after the lifted mask mandate. In Q1, the prescription market for cephalosporin antibiotics soared 88.8% in 3 years compared to Q1 2021. Cefaclor, the leading cephalosporin antibiotic, has recently grown significantly after the prescription market contracted in the early days of the COVID-19 outbreak. The outpatient prescription market for Cefaclor shrank by 23.2% from KRW 31.8 billion in Q1 2020 to KRW 24.4 billion in Q1 2021. However, from the end of 2021, the prescription market expanded at a rapid pace. In Q1, Cefaclor’s prescription size was KRW 40.6 billion, which is a 66.4% rise in 3 years from Q1 2021. Daewoong Bio was the dominant player in the Cefaclor market. Prescriptions of Daewoong Bio's Ceclor in Q1 was KRW 3.4 billion, up 20.1% YoY. Prescriptions of Ceclor have nearly doubled in 3 years from 1.8 billion won in Q1 2021. Prescription sales of Withus Pharamceutical’s Withus Cefaclor have increased sixfold from KRW 500 million in Q1 last year to KRW 2.8 billion in a single year. Other companies in the cephalosporin market, such as HLB Pharma, Mothers Pharmaceutical, Celltrion Pharm, Samsung Pharm, and Theragen Etex, generated over KRW 1 billion in prescriptions in Q1. Cephalosporin antibiotics have recently emerged as a new cash cow for pharmaceutical companies due to such a significant increase in demand. Many pharmaceutical companies had been considering withdrawing from the cephalosporin antibiotics business due to the declining market rising raw material costs and low drug prices. However, with the recent increase in demand, pharmaceutical companies are rather facing an urgent need to secure more supply. Daewoong Bios, which has been leading the market for cephalosporin antibiotics, announced last year that it would invest KRW 50 billion to build a new plant dedicated to the production of cephalosporin antibiotics. The current cephalosporin antibiotics plant in Seongnam, South Korea, will cease operation after this year due to outdated facilities. Once the new plant is completed, the company plans to further expand its antibiotic business. Separation of factories has become mandatory for the production of cephalosporin antibiotics since 2011. Since separate manufacturing facilities are required for its production, not many companies own dedicated facilities. One pharmaceutical company official said, "Cephalosporin antibiotics are a representative drug supplied through active consignment agreement. With the recent surge in demand, we have been struggling to secure sufficient supplies from our consignees.”
- Company
- Another petition for reimb listing of TNBC drug 'Trodelvy'
- by Eo, Yun-Ho May 07, 2024 05:50am
- Gilead Science Korea’s Trodelvy (Sacituzumab govitecan). There is now yet another petition requesting reimbursement listing of the new breast cancer drug 'Trodelvy'. There was already a previous posting with 50,000 votes in January of last year. It appears that another petition was posted because it has been three months without any updates. A petitioner, who introduced themselves on the national petition platform on May 2nd as a stage 4 triple-negative breast cancer (TNBC) patient, pleaded for support in need of Trodelvy reimbursement, stating, "The tumor is growing too fast. I earnestly request the community to participate to extend the treatment option to me as a stage 4 patient." There has not been any updates regarding Gilead Science Korea’s Trodelvy (Sacituzumab govitecan), a treatment of triple-negative breast cancer (TNBC), being considered for the Drug Reimbursement Evaluation Committee (DREC) after the clearance of the Cancer Disease Review Committee of the Health Insurance Review and Assessment Service (HIRA) in November of last year. Trodelvy is the first Antibody-Drug Conjugate (ADC) composed of a monoclonal antibody that binds to the cell surface antigen Trop-2 and a DNA TOP1 inhibitor payload called 'SN-38,' which destroys cancer cells. It received approval as a treatment for adult patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, including at least one for metastatic disease, from the Ministry of Food and Drug Safety (MFDS) in May last year. Although Trodelvy is the only drug to have demonstrated improvements in overall survival period among those used for second-line treatment or more of metastatic TNBC, it is stalled at getting the reimbursement listing due to cost-effectiveness evaluation. One factor that can anticipate reimbursement coverage is recognition for innovativeness, as seen in the case of Enhertu (trastuzumab deruxtecan), which was listed in April and received a benefit from the government based on its ICER value. Whether Trodelvy will be considered for the upcoming DREC review is to be watched. Meanwhile, Trodelvy demonstrated its clinical effectiveness through the Phase 3 ASCENT clinical study. It has shown a 49% reduction in the risk of death and a 57% improvement in progression-free survival (PFS) in adult patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who have received two or more prior systemic therapies, including at least one for metastatic disease, compared to patients who received single-agent chemotherapy (TPC, Treatment of Physician’s Choice). These effects were observed regardless of the presence of brain metastasis.
- Company
- Hyperparathyroidism drug Orkedia may be reimb in Q3
- by Eo, Yun-Ho May 07, 2024 05:50am
- ‘Orkedia,’ a drug for secondary hyperparathyroidism, has entered its final gateway to insurance reimbursement in Korea. According to industry sources, Kyowa Kirin Korea recently entered into pricing negotiations with the National Health Insurance Service for Orkedia (evocalcet), a new drug for secondary hyperparathyroidism that can develop in patients with chronic kidney disease on dialysis. If the company and the authorities reach an agreement within the 60-day negotiation deadline, the drug could be launched with reimbursement in Q3 this year. The drug passed the Health Insurance Review and Assessment Service's Drug Reimbursement Evaluation Committee last month after receiving domestic approval in November last year. Orkedia is a calcimimetic agent and acts on calcium receptors on the surface of parathyroid cells to inhibit excessive parathyroid hormone (PTH) secretion, thereby reducing serum parathyroid hormone levels Secondary hyperparathyroidism is a condition in which excessive secretion of parathyroid hormone persists due to hypocalcemia caused by decreased kidney function, leading to complications such as bone disease. Orkedia is expected to help expand treatment options for patients who are taking existing medications for secondary hyperparathyroidism. Meanwhile, Kyowa Kirin acquired the rights to cooperative research, develop, market, and manufacture Orkedia in Japan and some parts of Asia with Mitsubishi Tanabe in March 2008 and has since been developing the drug.
- Company
- Fate of Retevmo’s reimb in Korea receives attention
- by Eo, Yun-Ho May 03, 2024 06:00am
- No news has been heard of ever since its drug pricing negotiations broke down in August last year. However, RET inhibitors are a hard-to-forego treatment option for a small number of lung cancer patients. There are only 2 RET-targeted therapies – ‘Gavreto (pralsetinib),’ which Roche Korea introduced from Blueprint Medicines, and ‘Retevmo (selpercatinib’ by Lilly Korea – currently approved in Korea. Both drugs have attempted insurance reimbursement in Korea but were denied reimbursement last year. Adding Roche's market withdrawal for Gavreto, it would be hard for Gavreto to be listed for reimbursement in Korea. The case is not much better for Retevmo either. Retevmo, which was approved in Korea in March 2022, failed to pass the Health Insurance Review and Assessment Service Cancer Disease Review Committee (CDDC) review in May of the same year, but then passed the CDDC review in November and finally passed the Drug Reimbursement Evaluation Committee in May last year. After passing the DREC review, the company entered into drug price negotiations with the National Health Insurance Service in June, raising expectations on Retevmo’s reimbursement. However, the two ultimately failed to reach an agreement. In fact, it was the only news of a drug pricing negotiation failure reported in the past year. As a result, it has become unclear whether or not anticancer drugs targeting RET mutations will ever be covered in Korea. However, there has been one glimmer of hope - Phase III results of Retevmo that were released in October last year. Results from Phase III trials on Retevmo - LIBRETTO-431 and LIBRETTO-531 - were presented at the European Society for Medical Oncology Annual Congress (ESMO 2023) last year. The results were published in the internationally recognized New England Journal of Medicine (NEJM) along with the congress presentation. The LIBRETTO-431 trial presented at the meeting compared Retevmo with platinum-based chemotherapy±pembrolizumab as a first-line treatment in patients with advanced or metastatic RET fusion-positive NSCLC. Key findings in the trial showed that in the ITT-pembrolizumab population, the median progression-free survival (PFS) by an independent centralized review committee (BICR) was 24.8 months in the Retevmo arm, and 11.2 months in the control arm, with a hazard ratio of 0.465. The overall response rate (ORR) by BICR was 83.7% in the Retevmo arm, which was statistically significantly higher than the 65.1% in the control arm. Retevmo was granted conditional authorization for conducting a Phase III trial and had trouble during the registration process as the authorities requested the company for data equivalent to a Phase III trial due to its lack of a Phase III trial. This led to criticism about the evaluation criteria for conditionally approved drugs that were granted fast-track review at the time. Retevmo, which applied for fast-track status under the accelerated review system, had been in reimbursement discussions for about a year and a half to no avail. This time, however, the company has secured solid Phase III trial results. Now it remains to be seen whether Lilly will be willing to try again and whether the government will be willing to reimburse the RET inhibitor. In 2020, Retevmo was approved as the first treatment option for cancer patients with RET alternations in the US after the FDA reviewed the drug through the Accelerated Approval and Priority Review pathway and granted the Breakthrough Therapy & Orphan Drug Designation.
- Company
- Roche Korea appoints Ezat Azem as new general manager
- by Son, Hyung-Min May 03, 2024 05:52am
- Ezat Azem, new General Manager, Roche Korea Roche Korea announced that it had appointed Ezat Azem as the new general manager, effective as of May 1. The new GM first joined Roche’s Israel subsidiary in 1997, after which he served nearly 27 years in the group as a marketing division leader in Romania, Slovenia, and other countries around the world, contributing to the launch and growth of the company’s key products. From July 2019 to most recently, Azem has served as General Manager of Roche Greece. Azem was recognized for his excellent people leadership and expertise, particularly for successfully leading the strategic portfolio expansion in the Oncology, Hematology, and Specialty Care business units. In addition, during his service as GM of Roche Greece, 8 drugs were approved for reimbursement, improving patient access to new drugs in the area. Azem placed great emphasis on partnerships between the private, public, and academic sectors, and helped Roche fulfill its role as a trusted partner in building Greece's healthcare system. This has resulted in significant achievements, including bringing patients, the medical community, and companies together to ensure that patients receive healthcare closer to home. Azem graduated from the Hebrew University, where he majored in Medical Sciences. He later obtained an MBA from Tel Aviv University and completed executive education programs at INSEAD (Institut Européen d'Administration des Affaires) Business School in France and London Business School (LBS) in the U.K. The new GM said, “I am delighted to be joining Roche Korea, a country known for its excellent healthcare infrastructure. I look forward to contributing to improving the health of patients in Korea by introducing Roche’s innovative new drugs and personalized treatments, drawing on my various expertise.”
- Company
- 'Leclaza’s 5 counts of clinical results' to be showcased
- by Son, Hyung-Min May 02, 2024 05:53am
- It has been noted whether new anti-cancer candidates from the pharmaceutical and biotech industry in South Korea will be competitive on a global setting. According to industry sources on April 2nd, various Korean pharmaceutical companies, including Yuhan, HLB, and GI Innovation, will attend the 2024 American Society of Clinical Oncology (ASCO 2024) Annual Meeting, which will take place on 31st for five days in Chicago, United States. The AACR is the world’s largest cancer academic conference, at which 400 companies from 120 countries gather annually to present their latest clinical data. Yuhan-Janssen will disclose their major clinical data of Leclaza+Rybrevant Leclaza+Rybrevant combination therapy to be presented at ASCO 2024. Yuhan and its partnering company, Janssen, will present five counts of clinical data results related to Leclaza and Rybrevant combination therapy. Notably, the PALOMA 2,3 trial, which evaluated the effectiveness of the subcutaneous (SC) formulation of Leclaza and Rybrevant, is to be watched. As shifts toward SC formulations gain popularity in anti-cancer immunotherapy, such as Keytruda, Opdivo, and Tecentriq, targeted anti-cancer agents follow suit. Conventional anti-cancer agents are primarily intravenous (IV) therapy, and the administration takes more than one hour. Anti-cancer agents of SC formulation are expected to improve patient convenience since they can significantly reduce the administration duration to within 10 minutes. Phase 3 PALOMA-3 study has evaluated the efficacy and safety of SC formulation Leclaza and Rybrevant therapy compared to IV formulation Leclaza and Rybrevant combination therapy in patients with EGFR positive non-small cell lung cancer (NSCLC) who have failed previous treatments. Furthermore, the Phase 2 PALOMA-2 sutdy evaluated the effectiveness of SC formulation Rybrevant and Leclaza therapy in patients with EGFR-positive NSCLC who have not been treated before. It is to be watched whether the combination therapy of SC formulation can address the concerns regarding infusion-related reactions (IRR) side effects identified in the MARIOSA study. Also, the second analysis results of the Phase 3 MARIPOSA clinical study will be disclosed. These results are from testing the effectiveness of the Rybrevant and Leclaza combination therapy, with interim analysis results for progression-free survival (PFS) and OS previously disclosed in last year’s European Society for Medical Oncology Annual Meeting (ESMO 2023). Results related to high-risk patients with a specific biomarker are anticipated to be presented at this year’s ASCO. This study has evaluated the treatment’s efficacy and safety in comparison to its competitor, Tagrisso by AstraZeneca. Additionally, the clinical results of CHRYSALIS-2 study, evaluating the Leclaza and Rybrevant combination therapy in patients with NSCLC harboring atypical EGFR mutations, will be disclosed. This will be presented by Byoung Chul Cho (Director of the Lung Cancer Center at Yonsei Cancer Hospital), who directed the MARIPOSA research. The clinical results of the Leclaza and Rybrevant combination therapy in patients with EGFR-mutated NSCLC who have CNS diseases will also be disclosed. HLB to disclose additional results of rivoceranib in treating liver cancer…to showcase anti-cancer immunotherapy GI-102 Korean pharma and biotech companies, including GI Innovation, ABL Bio, NeoImmuneTech, and Qurient, to present their results at ASCO 2024. HLB will present the results of survival duration tracking for liver cancer patients at this conference. Rivoceranib is an oral targeted anti-cancer agent that inhibits vascular endothelial growth factor receptor 2 (VEGFR2), which is involved in tumor angiogenesis. Rivoceranib and camrelizumab combination therapy is currently anticipated to receive approval from the U.S. FDA. In a previously disclosed Phase 3 study of CARES-310, the combination therapy recorded an OS of 22.1 months in patients with liver cancer. The final analysis data of OS will be disclosed at ASCO 2024. GI Innovation will present the Phase 1/2 results of its anti-cancer immunotherapy candidate, GI-102, in metastatic solid cancer. This novel candidate product targets CD80 and interleukin (IL-2), targeting tumors and immune cells. According to interim results from the phase 1/2 clinical trial, GI-102 demonstrated response rates in cancer types unresponsive to conventional anti-cancer immune immunotherapy. Notably, an increase in response rates was observed in melanoma patients who were unresponsive to anti-cancer immunotherapy, with confirmation of proliferation in NK cells and T cells. ABL Bio will present its Phase 1 clinical result of ABL503, a bispecific antibody candidate. ABL503 is a cancer immunotherapy that simultaneously targets PD-1 and 4-1BB. According to the Phase 1 clinical trial result, one instance of complete response (CR) and three instances of partial remission (PR) were confirmed in ovarian cancer patients administered with ABL503. Phase 1 trials of ABL503 are being conducted in 6 agencies in the United States and 3 agencies in South Korea, related to dosage escalation and expansion parts. Once the dosage is determined, the company aims to set the optimal target among solid cancers. NeoImmuneTech will present poster presentations of preclinical data for its NT-I7, an anti-IL-7, in combination with the anti-cancer immunotherapy Keytruda for the treatment of solid cancer. This clinical trial evaluated the efficacy and safety of the combination therapy in 48 patients with pancreatic cancer and 50 patients with microsatellite stable (MSS) colorectal cancers. Qurient will showcase the interim result of the Phase 1 study for the CDK7 inhibitor Q901. Q901 has an underlying mechanism of inhibiting CDK7, a key molecule in regulating the cell cycle. This inhibits DNA damage repairs and increases gene instability, ultimately leading to apoptosis of cancer cells. Qurient is currently conducting a Phase 1/2 trial to evaluate the safety and efficacy of Q901 as a potential anti-cancer treatment in patients with progressive solid cancer. This trial is being conducted in the United States and South Korea.
- Company
- Yuhan earns KRW253.2B through licensing-out deals in 5 yrs
- by Chon, Seung-Hyun May 02, 2024 05:52am
- Yuhan Corp has secured a solid source of revenue with technology licensing fees. Billions of won in profit have been steadily flowing in every quarter through technology licensing deals, the investing company’s development progress, and milestone payments. Yuhan Corp’s cumulative technology fee over the past 5 years amounted to KRW 253.2 billion. According to the company's report on the 2nd, the company's technology fee revenue in Q1 was KRW 2.5 billion. This is down 64.4% from KRW 7.2 billion in the same period of the past year, but it has been 5 consecutive quarters the company has been collecting revenue since the Q1 in the past year. Yuhan’s quarterly technology fee revenue (Unit: KRW million, Source: Yuhan Corp) The CDMO technology fee it had received from its subsidiary Add Pharma played a major part in the company’s Q1 revenue in Q this year. Addpharma specializes in the development of Incrementally Modified Drugs (IMD). In 2022, Addpharma developed a rosuvastatin-ezetimibe combination drug and licensed it to Jeil Pharmaceutical and GC Biopharma. Yuhan acquired Addpharma in 2017 for KRW 3 billion and invested an additional KRW 7 billion in 2022. As of the end of last year, the company held a 67.7% stake in Addpharma. The company also received a portion of the technology fee from Boehringer Ingelheim in the first quarter. In July 2019, the company licensed out YH25724, a candidate drug for non-alcoholic steatohepatitis (metabolic dysfunction-associated steatohepatitis, MASH), to Boehringer Ingelheim for up to USD 870 million. Under the terms of the agreement, a non-refundable upfront payment of USD 40 million was made. In November 2021, YH25724 entered Phase 1 clinical trials, and the company earned an additional USD 10 million in milestone payments. Yuhan Corp has been receiving technology fee revenue since 2018 when it began exporting new drug technology in earnest. In July 2018, the company transferred its YH14618 technology for degenerative disc disease to Spine Biopharma in the US. The company received an upfront payment of USD 650,000 and a guarantee of milestone payments of USD 217.5 million based on development, approval, and sales. In November 2018, Yuhan Corp licensed out its anti-cancer drug Leclaza to Janssen Biotech. The total value of the agreement, including a non-refundable upfront payment of USD 50 million, amounted to USD 1.25 billion. In January 2019, the company entered into an agreement with Gilead Sciences to license and co-develop a drug candidate that acts on two targets for the treatment of nonalcoholic steatohepatitis (NASH). The deal includes an upfront payment of USD 15 million and milestone payments of USD 777 million depending on development, approval, and sales. In August 2020, the company entered into a licensing agreement with the US company Processa Pharmaceuticals to license out YH12852. YH12852 is a small molecule drug in development for the treatment of functional gastrointestinal (GI) disorders (FGID). The company received USD 2 million in Processa Pharmaceuticals’ common stock as a non-refundable upfront payment. Since then, the company has been receiving additional payments due to progress in the development stage of its licensed out candidates. In April 2020, the company received a milestone payment of USD 35 million from Janssen. At the time, Janssen paid the additional milestone payment to Yuhan Corp after initiating a clinical trial for amivantamab and Leclaza combination. In November 2020, Janssen paid an additional milestone of USD 65 million to Yuhan Corp after starting subject recruitment for its trial. With the USD 50 million in upfront payment, the company has secured a total of USD 150 million through licensing deal payments with Leclaza. Additional milestone payments are expected once ‘Leclaza receives U.S. approval. Janssen filed a New Drug Application (NDA) with the U.S. Food and Drug Administration (FDA) late last year for the combination of Rybrevant and Lexarza for EGFR-positive non-small cell lung cancer. From Q1 2020 to Q2 2021, the company recognized over USD 10 billion as technology fee revenue for 6 consecutive quarters. In Q4 2020, the company recognized a technology fee revenue of KRW 77.7 billion. In the 5 years from 2020 to Q1 this year, the total technology fee revenue recognized by the company amounted to KRW 253.2 billion.