- LOGIN
- MemberShip
- 2025-12-22 21:25:44
- Company
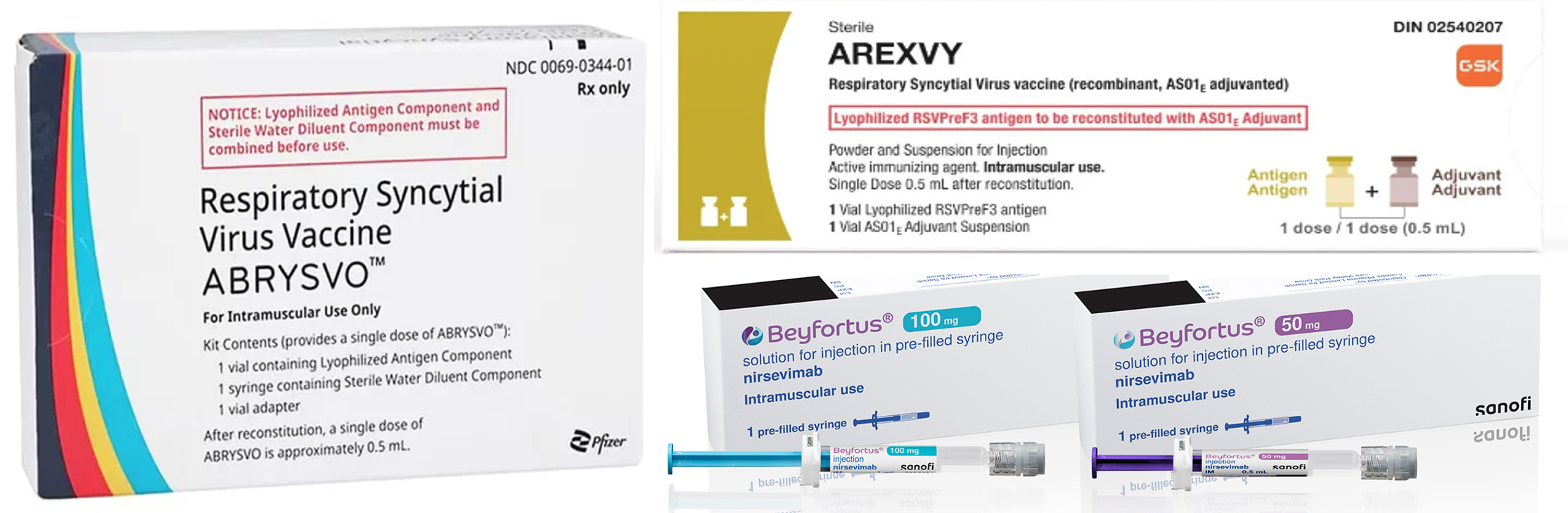
- Global RSV vaccines emerge…K-pharma enters clinical trials
- by Son, Hyung-Min May 20, 2024 05:42am
- The RSV vaccine Beyfortus, developed by Sanofi and AstraZeneca, GSK’s Arexvy, and Pfizer’s Abrysvo. Competition is expected to intensify as new drugs emerge in the respiratory syncytial virus (RSV) market. Sanofi received approval for its RSV vaccine and is the third company to acquire the approval, following GSK and Pfizer. Furthermore, Moderna’s messenger RNA (mRNA) vaccine awaits the FDA approval. In South Korea, EuBiologics has entered the Phase 1 trial. According to industry sources on the 20th, the RSV vaccine Beyfortus, developed by Sanofi and AstraZeneca, received approval in South Korea on April 30th. Consequently, Beyfortus has become the first preventative RSV vaccine targeting young children to obtain domestic approval from the regulatory institution. Last year, GSK’s Arexvy and Pfizer’s Abrysvo were launched overseas, but they have yet to start acquiring domestic approval. RSV is a virus that causes pneumonia and bronchiolitis. Although RSV can be infected at any age, young children are particularly prone to infection. It is the common cause of hospitalization for young children, with a high disease incidence, and 90% of young children worldwide are infected before 2 years. When infected, the symptoms are similar to the common cold. Certain young children experience worsening symptoms, having lower respiratory symptoms such as pneumonia and bronchiolitis. In South Korea, RSV Synagis Inj, an antibody directed to RSV, is available. However, it has the disadvantage of multiple injections due to short-lasting in the body. Moreover, Synagis can only be administered to patients with certain diseases. With the long-lasting Beyfortus, healthy children less than 24 months can be vaccinated. The efficacy and safety of Beyfortus were confirmed in MELODY clinical trials against medically attended Lower Respiratory Tract Infection (LRTI) caused by RSV through 150 days after the treatment. In clinical trials, Beyfortus reduced the RSV LRTI infection rate to 74.5% in full-term infants and late preterm infants. The Beyfortus effect was 70.1% in preterm infants. Furthermore, the NIRSE-Gal interim analysis result, which was the basis for the European real-world evidence (RWE), has shown that the Beyfortus administration reduced the hospitalization rate by 82% in infants less than 6 months compared to non-vaccinated infants. RSV circulates between October and March every year. Sanofi and AstraZeneca are producing Beyfortus before the RSV season and plan to distribute most vaccine stocks before October. Moderna completes clinical trials and waits for FDA approval...EuBiologics enters clinical trials in South Korea as the first and only Moderna’s RSV vaccine will receive the decision for the FDA approval within this month. Moderna’s mRNA-1345 vaccine is developed to prevent RSV in adults over 60. When approved, it will be the third RSV vaccine that targets adults, following Arexvy and Abrysvo. The ConquerRSV trials, which evaluated the safety and efficacy of Moderna’s RSV vaccine, involved about 37,000 adults 60 years or older in 22 countries, including the United States. They were conducted as randomized, double-blind, and placebo-controlled studies. The interim analysis was based on two definitions of RSV-LRTD defined as either two or more symptoms, or three or more symptoms of disease. According to the clinical results, mRNA-1345 demonstrated vaccine efficacy of 83.7% in RSV-LRTD defined as two or more symptoms, and all primary endpoints were met. Adverse reactions of mRNA-1345 were mild or moderate, and the most common adverse reactions reported in the mRNA-1345 group were injection site pain, fatigue, headache, myalgia, and arthralgia. Moderna expects approval this month and plans to launch its first RSV vaccine to the market by the second half of the year. Additionally, EuBiologics has entered full-scale clinical trials in South Korea. EuBiologics’ euRSV vaccine under development has recently started administration in three RSV patients in Korea University Guro Hospital. The clinical trials involve patients randomly assigned to a placebo group, EuRSV low-dose group, or high-dose group, and tolerability and the safety will be evaluated. EuRSV is a recombinant protein subunit vaccine that combines an RSV F protein antigen with immunostimulants. In 2017, EuBiologics acquired immune modulation technology (EuIMT) from KIST. EuBiologics emphasizes that the manufacturing process of EuIMT is simple compared to GSK’s immunostimulant technology, MPL(monophosphoryl lipid). Consequently, the company expects that vaccine mass manufacturing will be possible at a low cost. After starting a phase 1 trial in South Korea, EuBiologics aims to enter global phase 2/3 trials through its U.S. subsidiary EUPOP Life sciences, which has North America and Europe licenses.
- Company
- ‘Much work remains to be done for new drug access in Korea'
- by Eo, Yun-Ho May 20, 2024 05:42am
- Kyung-Eun Bae (Kay Bae ), Chair of the Korean Research-based Pharmaceutical Industry Association Kyung-Eun Bae (Kay Bae, 53) has risen to become the 'center' of the pharmaceutical industry. After being appointed as the general manager to lead subsidiaries including Korea, New Zealand, and Australia of the Sanofi Group, Bae was also appointed Chair of the Korean Research-based Pharmaceutical Industry Association (KRPIA) Being appointed to head the association representing multinational pharmaceutical companies in Korea along with Sanofi Group’s major Asia-Pacific countries, Bae has become a key figure in communicating with the industry and regulatory authorities, including Sanofi headquarters and the Korean government. Bae’s appointment came at the busiest time. Bae has received an offer every time the KRPIA Chair position became open, but she has turned down the offer up to now. And in a situation where her scope of work became broader than ever before, she accepted the long-declined offer. Serving as KRPIA Chair at the busiest time of her career, there had been concerns amid rising expectations about whether Bae would be able to do her part and perform both roles well. As a control tower, KRPIA has been working to make new drugs more affordable than ever before. Making proposals to reform the drug pricing system and engaging in government communications for its support has become a key role of the association. In her two roles, Bae will be overseeing two of Korea’s regular drug pricing reference countries - New Zealand and Australia – at Sanofi, and be determining KRPIA’s key message and strategy on behalf of the industry. In other words, Bae may be best positioned to make the most appropriate policy recommendations. Dailpharm met with Bae to find out more about her new roles. - Your appointment as KRPIA Chair came as a surprise because I thought you were going to turn down the position again. I became the head of Sanofi Australia and New Zealand and the Chair of KRPIA at the same time. I was hesitant at first because I didn't think I had the capability yet. However, I have always felt that I should contribute to the association's development because KRPIA's mission aligns with the values that global pharmaceutical companies deem important. I thought this year was the right time to take on the role. It seems to be that time of life for me when I’m destined to work. So I plan to dedicate myself to the work at hand while taking care of my physical health. I apologize for the unpredictability (laughs). - This is a very important time for multinational pharmaceutical companies. Looking at the government's policy direction, although there are still some policies that add pressure, there are also positive signals, such as the recent plan to compensate for the value of innovative new drugs. The phrase 'new drug value recognition' appeared for the first time in the 'Second Comprehensive National Health Insurance Plan' released by the government this year. It's a good start and an encouraging achievement as it lays the groundwork for improving patient access, including recognizing the innovativeness and value of new drugs and strengthening coverage for seriously ill patients. However, it is important to come up with concrete measures that are effective in practice so that new drugs can be supplied to patients quickly and lead to a virtuous cycle of R&D. -What are the KRPIA's recommendations to the government to address the practical issues you mentioned? There are two main areas I would like to point out. First, Korea is still too late in introducing new drugs. Statistics show that the percentage of new drugs introduced in Korea within 1 year of initial global (US, EU, etc.) approval is only 5%, which is very low compared to Japan and the US. Whether a new drug can actually be used by patients is most important. To address this issue, Korea has a Global Innovative Products on Fast Track (GIFT) system in place. However, we need to check whether the system is really shortening the review period and where the bottlenecks are. I think we first need to come up with a solution for drugs that are in urgent need of introduction. The second is in recognizing the value of new drugs. Companies are motivated when the value of new drugs is recognized, which in turn generates profits and creates a virtuous cycle of R&D. This ultimately requires a significant change in drug pricing policies, including a dramatic improvement in ICER values. When global pharmaceutical companies consider whether to invest in R&D in China, Japan, Southeast Asia, and Singapore, among other countries, they have to prioritize investing in countries with a strong R&D reputation. From an industrial perspective, this is why we need to move to reinforce Korea’s R&D competitiveness. Also, due to limited national health insurance finances, we need to take a look at the expenditure structure. In terms of the proportion of new drugs in drug expenditures, new drugs account for 60 to 70% of all drug expenditures abroad, but they account for less than 10% in Korea. We need to reorganize the inefficient systems that allow polypharmacy and clinic shopping. This way, we can budget for new drugs without putting a strain on insurance finances. -It was also surprising to see KRPIA voice the need to improve Korea’s expenditure structure. structure. Multinational pharmaceutical companies have been cautious in speaking out on the issue because the domestic market still has a strong tendency to look at new drugs as being from multinational companies, and generic drugs from domestic companies. Many were surprised that we released data on Korea’s drug expenditure structure (and the proportion of new drugs) last year. However, the government should be encouraging domestic companies to develop new drugs now. From the PE exemption system to ICER valuations, everything is connected, so KRPIA plans to communicate with the government on the issue whenever there is an opportunity. -It was also unexpected that KRPIA would request the government to disclose the discussions and results of committee reviews (such as those by the Pharmacoeconomic Evaluation Subcommittee) that new drugs undergo in the reimbursement process. Of course, securing the transparency of the reimbursement process may be a cautious matter for companies due to each company’s strategic secrets and so forth. But in the big picture of healthcare, patients are waiting. Information sharing is really important for decision-making. Transparency in that area is a win-win for the companies. I think this is why the government has been responding to many of the requests. -The government's efforts to strengthen post-listing control of drugs are also expected to be in full swing this year. Policies such as those that utilize real-world data (RWD) and switching the pharmacoeconomic evaluation exemption system to a temporary deferral of evaluation system may be of concern and dissatisfaction to KRPIA. I think post-listing control is necessary in part to manage the overall insurance finances. However, Korea’s post-management system is not integrated and is too fragmented, including the PVA (price-volume agreement), which often results in redundant drug price reduction mechanisms From the company's point of view, the system needs to be simplified to be predictable. Currently, the government operates a follow-up system that focuses only on drug prices, such as PVA, which creates a high administrative burden. I would like to see a system that takes into account the structural aspects, such as strengthening health insurance coverage for severe diseases over mild diseases. There seems to be too much focus on just reducing drug prices. Strengthening post-listing control without improving the system and policies for rapid new drug registration in Korea will eventually lead to another decline in access to new drugs. KRPIA is preparing a report on the problems of the post-listing control measures and suggestions for their improvement that we plan to propose to the government based on the views of professionals with extensive experience in the field.
- Company
- Welireg can be prescribed in general hospitals in Korea
- by Eo, Yun-Ho May 20, 2024 05:41am
- Welireg, a rare anticancer drug for a small number of patients, can now be prescribed in general hospitals in Korea. According to industry sources on the 17th, MSD Korea’s oral hypoxia-inducible factor-2 alpha (HIF-2α) inhibitor, ‘Welireg (belzutifan) passed the drug committees (DCs) of various medical centers in Korea including the National Cancer Center, Samsung Medical Center, and Asan Medical Center. Welireg was designated an orphan drug in Korea for the treatment of Von Hippel-Lindau disease in January last year, then formally approved in May of the same year. Specifically, the drug is indicated for the treatment of adult patients with VHL disease who require therapy for associated renal cell carcinoma (RCC), central nervous system (CNS) hemangioblastomas, or pancreatic neuroendocrine tumors (pNET), not requiring immediate surgery. As a HIF-2α inhibitor, Welireg reduces transcription and expression of HIF-2α target genes associated with cellular proliferation, angiogenesis, and tumor growth. The drug’s efficacy was demonstrated in the open-label Study 004 trial, which investigated 61 patients with VHL-associated RCC who were diagnosed with at least one measurable solid tumor localized to the kidney. Patients enrolled in the trial had other VHL-associated tumors including CNS hemangioblastomas and pNET. The major efficacy endpoint of the clinical trial was the overall response rate (ORR) in patients with VHL-associated RCC as measured by radiology assessment using RECIST v1.1 as assessed by an independent review committee (IRC). Additional efficacy endpoints included duration of response (DoR) and time to response (TTR). In the study, Welireg showed an ORR of 49% in patients with VHL-associated RCC. All responses were partial responses. The median DoR had not yet been reached, and the DoR among responders who were still responding after at least 12 months was 56%. Median TTR was 8 months. Also, in patients with VHL-associated CNS hemangioblastomas, Welireg showed an ORR of 63%, with a complete response rate of 4% and a partial response rate of 58%. Meanwhile, Welireg was additionally approved for a kidney cancer indication in the US. Its efficacy for the indication was confirmed in LITESPARK-005, a trial conducted on patients with unresectable locally advanced or metastatic clear cell renal cell carcinoma (RCC) that had progressed following both a PD-1 or PD-L1 checkpoint inhibitor and a VEGF-TKI. Results showed that Welireg improved progression-free survival (PFS) and reduced the risk of disease progression or death by 25% compared to everolimus in patients with advanced renal cell carcinoma who received a PD-1 or PD-L1 immune checkpoint inhibitor and a VEGF receptor-targeted therapy, either sequentially or in combination.
- Company
- 'Imfinzi' shown to be effective for liver cancer
- by Son, Hyung-Min May 17, 2024 05:47am
- Hong Jae Chon, Professor of the Department of Hemato-Oncology at CHA Bundang Medical Center Imfinzi, an immunotherapy treatment for cancer, demonstrated effectiveness for liver cancer in addition to bile duct cancer. Consequently, it is expanding its uses in gastrointestinal cancer. AstraZeneca confirmed the effectiveness of Imfinzi in combination therapy with Imjudo, which targets CTL-A4, as a first-line treatment for liver cancer. The medical experts analyzed that this combination therapy has given hope for extended survival in patients with liver cancer. AstraZeneca hosted a press conference at Grand InterContinental Seoul Parnas on May 14th to commemorate the launch of a combination therapy, Imfinzi plus Imjudo. AstraZeneca’s Imfinzi is an immunotherapy for cancer that targets PD-1. It has been launched as a combination therapy with Imjudo, which targets CTL-A4, for the treatment of liver cancer. In June of last year, Imjudo was approved as a combination therapy with Imfinzi for the first-line treatment of liver cancer. Through this launch, Imfinzi has expanded its treatment area in gastrointestinal cancer, including bile duct cancer and liver cancer. Imfinzi was approved as a first-line treatment for bile duct cancer last month. The HIMALAYA Phase 3 trial demonstrated the effectiveness of Imfinzi plus Imjudo combination therapy. The trial compared the efficacy and the safety profile of Imfinzi plus Imjudo combination therapy to Bayer’s Nexavar, enrolling 1,171 patients with unresectable liver cancer who are 18 and over and have no prior treatment history. As part of the treatment regimen, Imfinzi plus Imjudo was administered on day 1 of week 1, followed by Imfinzi monotherapy only for the next 4 weeks. Nexabar was administered twice per day. Clinical results showed that Imfinzi plus Imjudo combination therapy reduced the risk of death by 22% compared to Nexabar monotherapy. Imfinzi plus Imjudo combination therapy’s overall survival (OS) was 16.4 months, whereas that of Nexabar monotherapy was 13.8 months. The difference in OS between Imfinzi plus Imjudo combination therapy and Nexabar monotherapy expanded over time. At week 18 of treatment, the OS of Imfinzi plus Imjudo was 48.7%, and that of Nexabar was 41.5%. The difference in OS was maintained up to 3 years of treatment. For the safety profile, the rate of Grade 3 or Grade 4 treatment-related adverse drug reactions in Imfinzi plus Imjudo combination therapy was 25.8%, and that of monotherapy was 36.9%. The Imfinzi plus Imjudo combination therapy is expected to compete with Roche’s combination therapy of Tecentriq, an immunotherapy for cancer, and Avastin, a targeted anticancer agent. Previously, Tecentriq and Avastin also proved to improve OS compared to Nexabar. Hong Jae Chon, Professor of the Department of Hemato-Oncology at CHA Bundang Medical Center, said, “In addition to Imfinzi, we now have more treatment options for liver cancer with the emergence of Tecentriq combination therapy. The advantage of Imfinzi plus Imjudo combination therapy is reduced risk of bleeding. The risk of bleeding in patients with liver cancer is high due to decreased liver function. Because Imfinzi combination therapy has reduced risk of bleeding, endoscopic surgery is possible right away.” “Patients with liver cancer who have accompanying liver function reduction have a high mortality rate. However, Imfinzi does not worsen the liver function. Based on my experience, treatment outcome is better than expected,” Chon added. “If combination therapy reimbursement is approved, its use will increase. Currently, it will be used without coverage for patients requiring the therapy.”
- Company
- Ferring Korea appoints Min-Jung Kim as new GM
- by Son, Hyung-Min May 17, 2024 05:47am
- Min Jung Kim, new General Manager of Ferring Pharmaceuticals Korea On May 16, Ferring Pharmaceuticals Korea announced that the company appointed Min-Jung Kim as its new general manager, effective as of May 1. The new GM joined the company in 2021 as Chief Financial Officer (CFO). Kim has over 20 years of experience in finance, business development, SCM, IT, and human resources at global pharmaceutical and medical device companies, including Novartis Korea and Pentax Medical Korea. In 2022, Kim served as the Chief Financial Officer for the Northern Asia region, where she improved Ferring's financial circumstances and drove business growth based on her financial expertise and strategic planning capabilities She also has verified her management capabilities in Korea, Hong Kong, and Taiwan, establishing various brand portfolio strategies and execution plans and collaborating with various stakeholders to derive results. Kim is also committed to building an innovative organizational culture and processes that can enhance employee engagement and professionalism to improve performance at Ferring Pharmaceuticals Korea. Based on her strong leadership, she has transformed Korea’s traditional hierarchical organizational culture into an open and communicative culture, while developing data-driven insights for efficient decision-making and a productive organization that fosters a productive work environment for its employees. "I am excited to be leading Ferring Pharmaceuticals Korea, which has always strived to provide innovative treatments to patients suffering from various diseases under the corporate philosophy, ‘People come first,'" said Kim. "Based on the experience and know-how I accumulated in the industry, I will make my best efforts to strengthen the company’s capabilities, while solidifying relationships with patients and stakeholders and contributing to the company's sustainable growth."
- Company
- Repotrectinib receives orphan drug designation in Korea
- by Eo, Yun-Ho May 17, 2024 05:47am
- , The ROS1-targeted lung cancer drug repotrectinib received an orphan drug designation in Korea. The Ministry of Food and Drug Safety (MFDS) announced the designation in a recent announcement. Specifically, the indications repotrectinib received orphan drug designation for are as follows: ▲ treatment for patients with ROS1-positive locally advanced or metastatic non-small cell lung cancer (NSCLC) ▲ treatment for patients with solid tumors that have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion, and are locally advanced or metastatic or where surgical resection is likely to result in severe morbidity. Repotrectinib was first approved by the US FDA in November last year as a treatment for NSCLC. It is available under the brand name Augtyro. The NTRK gene fusion solid tumor indication is under review after being granted a fast-track designation in February this year. The drug’s efficacy was confirmed through the multinational Phase I/II TRIDENT-1 trial. Trial results showed that the primary endpoint, the objective response rate (ORR), was 79% for repotrectinib in 71 TKI treatment-naïve patients. Progression-free survival (PFS) was nearly double that of previous targeted therapies. The ORR was defined as the proportion of patients treated within a specific time frame who either had a decrease in tumor size (partial response) or no further signs of cancer (complete response). The median duration of response was 34.1 months. In 56 ROS1 TKI–naïve and chemotherapy-naïve patients, the objective response rate was 38%, and the median duration of response was 14.8 months. The trial also examined the drug’s effect in treating patients with resistance to existing targeted therapies. The 56 patients with resistance to existing therapies showed an ORR of 38% and a PFS of 9 months, and 17 patients who had acquired a baseline G2032R resistance mutation showed an ORR of 59% and a PFS of 9.2 months. The results of the TRIDENT-1 trial were published in the New England Journal of Medicine (NEJM) (IF 176.082), with Byoung-Chul Cho, Director of the Lung Cancer Center at Yonsei Cancer Hospital in Korea, participating as a corresponding author. ROS1-positive NSCLC accounts for 2% of all lung cancers. The standard of care is to use targeted anti-cancer therapies that target the mutated gene, including ‘crizotinib’ and ‘entrectinib.’ Meanwhile, repotrectinib is gaining attention as a next-generation drug.
- Company
- Chinese Pharmas show presence at BIO Korea 2024
- by Moon, sung-ho May 16, 2024 05:48am
- Chinese pharmaceutical companies are starting to enter the domestic pharmaceutical and biotechnology market in earnest. This was the main observation made by the Korean industry at ‘BIO Korea 2024,’ cohosted by the Ministry of Health and Welfare and the Korea Health Industry Development Institute. The activities of major Chinese pharmaceutical and biotech companies at this year's BIO Korea attracted industry-wide attention. Among the companies, WuXi Biologics, a Chinese company that has grown into a global antibody contract development and manufacturing organization (CDMO), received the most attention. It set up a booth at the front of the venue and actively communicated with visitors and representatives from domestic pharmaceutical and biotech companies. With its CDMO competitors, LONZA and FUJIFILM Diosynth Biotechnology (hereinafter “Fujifilm”), also setting up booths at the event, it was evident that the competition among global CDMOs to expand their territories had begun in earnest. The companies seem to be working to expand their CDMO business through cooperation with domestic pharmaceutical and biotech companies. On the other hand, domestic companies competing in the CDMO market, such as Celltrion and Samsung Biologics, did not open their booths at the event. One Chinese company that stood out was Pharmaron Clinical, a CRO and CDMO company. Although the company’s size is not as large as other CDMOs like Wuxi, but the company actively engaged in talks to collaborate with domestic companies. A representative of a biotech company that participated in the event said, "Chinese companies most actively participated in this year’s BIO Korea event. They seem to be actively working to increase partnerships with domestic pharmaceutical and biotech companies to produce their treatments.” In addition, BeiGene, which has emerged as a strong player in the global anti-cancer drug market, also drew attention for its active cooperation activities at BIO Korea. Rather than setting up a booth, the company participated in the conference and held its own business briefing to strengthen partnerships with domestic Korean biotech companies. At the ‘Bio‧Pharmaceutical Global Open Innovation Cooperation and Activation Strategy’ session of the conference, BeiGene’s key executives in charge of BeiGene Asia introduced the company’s strengths and actively engaged in communication with domestic companies. During the session, BeiGene emphasized its financial resources and clinical strengths as a benefit. In fact, BeiGene’s self-developed anti-cancer drugs, such as Brukinsa (zanubrutinib), Tevimbra (tiselizumab), and Partruvix (pamiparib), are generating sales not only in China but also in the global market. The anticancer drug Brukinsa has been approved in more than 70 countries worldwide and is approved by the US FDA for five indications. In the first quarter of this year, BeiGene’s sales reached USD 752 million (KRW 1.0288 trillion), according to the company's latest financial result that it had released the previous day. Adam Roach, Vice President and Head of Asia Pacific at BeiGene, said, “Our biggest strength is that we don't need a separate CRO because we run our own clinical trials. Rather than using a CRO, we use our own internal clinical team that consists of over 3,000 people. We conduct more than 130 clinical trials in-house in about 48 locations." In the following lecture, Evan Goldberg, Vice President of Business Development at BeiGene, said, “Global big pharma companies used to be interested in only Japan in the Asia-Pacific region, but this is not the case anymore. Asia-Pacific countries, including Korea, have entered the global pharma and bio ecosystem, which is evidenced by the fact that Korean pharma and bio companies have been making frequent global technology transfers in recent years." Goldberg added, "BeiGene is currently working with global big pharma companies like Amgen, BMS, and Novartis, in addition to small and medium-sized biotechs. Some of these companies are seeking to enter the Asian market through the partnership, while Asian companies seek partnerships with the dream of going global." The domestic pharma and biotech companies that participated in BIO Korea have also noted the growth of Chinese companies and agreed on the need to revisit the companies’ treatments. An official from a pharmaceutical and biotech consulting firm who requested anonymity, said, "BeiGene’s clinical strength is definitely attracting attention in the global market. In the past, it only had a large number of trial enrollees in China, but the company is now actively conducting clinical trials in the global market. Considering the recent clinical trials it has been announcing, it’s time for the company to reestablish its position not just in Asia but also in the global market.
- Company
- Xcopri’s cumulative revenue surpasses KRW 1 trillion
- by Chon, Seung-Hyun May 16, 2024 05:48am
- Xcopri, SK Biopharmaceutical's new epilepsy drug, is continuing to show strong growth in the US market. Since its launch, sales have continued to rise every quarter, with cumulative sales exceeding KRW 600 billion. When including the revenue it had collected by licensing-out Xcopri’s technology, the company has secured more than KRW 1 trillion with Xcopri alone. According to SK Biopharmaceuticals on Thursday, Xcopri recorded KRW 90.9 billion in US sales in Q1 this year. This is a 68.6% rise from the KRW 53.9 billion it had posted in Q1 last year and is the largest amount earned since the drug entered the US market. This is also a 17.0% increase in a single quarter, from the KRW 77.7 billion in Q4 last year. Quarterly U.S. sales of Xcopri (Unit: KRW 100 million, Source: SK Biopharmaceuticals) Xcopri, which contains ‘cenobamate,’ is a new drug for epilepsy that SK Biopharmaceuticals. SK Biopharmaceuticals had independently performed the whole course of Xcopri’s development and commercialization from early development to US Food and Drug Administration (FDA) approval. It is prescribed for the treatment of partial-onset (focal) seizures in adult patients. Its mechanism of action relieves seizure symptoms by simultaneously regulating two targets related to excitatory and inhibitory signaling, which are causes of epilepsy. The company received approval for its cenobamate under the brand name ‘Xcopri’ in November 2019, and has been selling the drug through SK Life Science since May 2020. SK Life Science has about 150 medical representatives. Epilepsy is a condition that is treated by a small number of focus specialists in the United States. Due to the small number of target physicians, the company was able to sell the drug directly in the U.S. with such a small sales force. SK Life Science’s marketing center, which consists of sales and marketing personnel, has experts with more than 20 years of experience in successfully launching and selling epilepsy treatments and major central nervous system drugs at Johnson & Johnson, UCB, and other leading companies in the U.S. CNS market. Since its U.S. launch, Xcopri has been setting new sales records every quarter. It generated its first revenue of KRW 2.1 billion in Q2 2020 and surpassed KRW 10 billion in Q1 2020. Its sales surpassed KRW 50 billion in revenue in Q1 last year and have reached nearly KRW 100 billion in quarterly revenue this year. Xcopri’s cumulative revenue in the US totaled KRW 621.7 billion. Since 2019, Xcopri has earned more than KRW 400 billion in technology fees. In February 2019, SK Biopharmaceuticals signed a licensing-out agreement with Switzerland's Arvelle Therapeutics to transfer technology on cenobamate for up to $530 million. At the time, SK Biopharmaceutical received an upfront payment of $100 million with no obligation of return. In October 2020, the company entered into an exclusive licensing agreement with Ono Pharmaceutical for Ono to develop and commercialize Xcopri in Japan. Under the agreement, SK Biopharmaceutical received an upfront payment of ¥5 billion with no obligation of return. In November 2021, SK Biopharmaceuticals licensed out 6 new central nervous system (CNS) drugs including cenobamate to the Chinese company, Ignis Therapeutics. Under the deal, SK Biopharmaceutical received an upfront payment of $20 million. Through the technology export, SK Biopharmaceutical also acquired 150 million shares of Ignis (share amounts to 44.9% including common stock). In December 2021, SK Biopharmaceutical signed a license deal with Endo Group for the commercialization of its epilepsy drug cenobamate across Canada. Under the deal, SK Biopharmaceutical received an upfront payment of CAD 20 million. Paladin Labs Inc, a Canada-based operating subsidiary of Endo, will be responsible for all commercial activities related to cenobamate in the region, including its release. Endo is a global healthcare company headquartered in Ireland. In July last year, SK Biopharmaceuticals signed a licensing out deal with the Brazilian pharmaceutical company Eurofarma Laboratorios SA for cenobamate. Under the agreement, SK Biopharmaceuticals will receive an upfront payment of USD 15 million and up to USD 47 million in milestone payments. Under the licensing out agreement, Eurofarma will be selling cenobamate in 17 Latin American countries including Brazil and Mexico In addition to upfront payments, the company has also received milestone payments upon cenobamate’s approval abroad. SK Biopharmaceuticals received USD 123.22 million from its European partner Angelini Pharma as milestone payments in 2022. Angelini Pharma (formerly Arvelle Therapeutics UK) has paid additional milestone payments after receiving marketing authorization for Xcopri from the European Commission in March 2021. SK Biopharmaceuticals’ cash inflow from upfront payments and further milestones from the technology transfer of cenobamate is USD 301.22 million and ¥5 billion. The company has secured about KRW 450 billion through upfront and milestone payments through technology transfer agreements with cenobamate. Combined with US sales, the single product had brought in over KRW 1 trillion. Xcopri’s sales growth has also improved SK Biopharmaceuticals’ performance. In Q1 this year, SK Biopharmaceuticals posted an operating profit of 10.3 billion won, a YoY turnaround and its sales rose 87.5% YoY to KRW 114 billion. An official from SK Biopharmaceuticals said, "This is the first time since its establishment that SK Biopharmaceuticals has achieved a surplus for 2 consecutive quarters, and it is significant that the profitability in Q1 was achieved entirely due to the growth of cenobamate’s sales in the US.”
- Company
- "Camzyos, reimbursement would benefit gov and patients"
- by Eo, Yun-Ho May 16, 2024 05:48am
- Hyung-Kwan Kim, Professor of the Department of Internal Medicine at Seoul National University Hospital The reason for delayed drug development in a particular disease typically falls into one of two. It’s either low awareness of the disease or challenges to drug development. Despite falling into these two categories, 'Camzyos (mavacamten)' was developed. It is a first-in-class targeted treatment option for obstructive hypertrophic cardiomyopathy (oHCM). oHCM is a rare heart disease characterized by thickened heart muscles that cause narrowing or obstruction of the left ventricular outflow tract (LVOT). Not only does it cause abnormality in heart structure, but it decreases heart function. The symptoms include shortness of breath, chest pain, dizziness, and fainting. It is a fatal disease that could cause cardiovascular complications and sudden cardiac death. Furthermore, there has been a lack of disease-modifying treatment besides short-term management of symptoms. Thus, the development of Camzyos, which works by significantly reducing cardiac myosin actin cross-bridge formation, was exciting news to doctors. However, the concern is whether it could be covered by insurance reimbursement. Although Camzyos was approved in South Korea in May of last year, it has not yet been covered by reimbursement. BMS is pursuing an application for the drug’s listing and is awaiting the review by the Drug Reimbursement Evaluation Committee (DREC) of the Health Insurance Review and Assessment Service (HIRA). Daily Pharm interviewed Hyung-Kwan Kim, Professor of the Department of Internal Medicine at Seoul National University Hospital, about Camzyos’ value and the necessity of reimbursement. -HCM diagnosis has increased following the expansion of reimbursement criteria for cardiac ultrasonography. Has the diagnosis rate increased in the real-world? What is the patient size of HCM in Korea? We don’t have accurate data for Korea yet, but the National Health Insurance Service (NHIS) data shows that the number of patients who are diagnosed with HCM has increased since 2010. Between 2010 and 2016, the number of HCM patients doubled. The increase in diagnosis rate may have been due to the routine health examination in Korea: cardiac ultrasonography can be added to the examination option. This supports the trend since the number of diagnoses has increased, particularly among patients over 50. -It seems that there is a low level of awareness among medical personnel regarding oHCM. In fact, due to the lack of effective treatments for HCM, awareness of the condition has been low, and research has not been very active. Even if someone showed signs of suspected HCM or received a diagnosis, the lack of available therapies meant that coordinated care across tertiary healthcare facilities was almost non-existent. Patients were typically advised to manage their symptoms and avoid strenuous exercise, as no competitive treatment options were available. -The development of Camzyos must be particularly meaningful. Previously, beta-blockers and calcium channel blockers were commonly used to treat oHCM. However, these medications do not target the pathophysiological mechanisms of oHCM, leading to limited effectiveness, and many patients do not experience significant improvement. Disopyramide was previously used but is no longer recommended due to its side effects. Furthermore, since it is not prescribed in South Korea, there are few options for treating oHCM. Medical professionals couldn't help but have high expectations when Camzyos became available. Initially skeptical, I found the clinical data remarkably convincing regarding effectiveness. Even data from patients treated with Camzyos abroad showed significant changes in cardiac ultrasonography findings. Among the seven patients under my care who received Camzyos, almost all showed improvement to the extent that it could be 100%, with effects evident within a month. -The clinical results of Camzyos demonstrated improved exercise capacity in oHCM patients. How significant is this result? Exercise capacity can be categorized into objective measures that can be quantitatively assessed and subjective measures that patients evaluate based on their experience. In clinical studies, Camzyos significantly improved both of these indicators. The subjective indicators of patients currently undergoing Camzyos treatment have also improved across the board. NYHA classification has improved by at least one level, with some cases improving from grade 3 to grade 1. In addition to exercise capacity, cardiac function can be evaluated by checking NT-proBNP levels through blood tests. Among the patients under care, those with elevated NT-proBNP levels at the start of Camgios treatment all experienced decreased NT-proBNP levels. Some patients saw remarkable effects, with NT-proBNP levels dropping from 2,000 to 3,000, within the normal range after just one month of Camzyos treatment. -Last year, the European Society of Cardiology (ESC) updated its guidelines for the management of cardiomyopathy for the first time in about 9 years since 2014, adding recommendations for the treatment of Camzyos. What do you think about this? It's promising. I had hoped it would be recommended as a first-line treatment, but ESC seemed to take a conservative approach. Considering the nature of ESC guidelines, where first-line recommendations are not often made right away, there is ample possibility for Camzyos to be recommended as a first-line therapy in the future. -It sounds like Camzyos could possibly be a first-line treatment. Yes. Considering the pathophysiological mechanism of oHCM, I believe it is important for Camzyos to become the first-line treatment option in the long term. This way, we can reduce the unnecessary time and medical expenses of patients who use ineffective medication for 2-3 months. -The concern is that Camzyos is still a non-reimbursed drug. There must be disappointments in the real-world. oHCM is associated with a higher risk of stroke because atrial fibrillation and aneurysms of the left ventricle are not uncommon even in younger age groups. In the case of a oHCM patient in his late 40s, who had no symptoms at all, an aneurysm was found in a screening test conducted every 2-3 years. Currently, in Korea, NOACs (new oral anticoagulants) cannot be used to prevent stroke with aneurysms alone, and warfarin must be used, which requires continuous monitoring when administering warfarin, which is very difficult. So, I explained to the patient that I thought oHCM caused the aneurysm and suggested surgery or treatment with Camzyos. In the end, the patient opted for Camzyos treatment, and within one month, what appeared to be a pericardial aneurysm improved, and the condition of the heart that had been obstructed improved. If he hadn't been treated for Camzyos, his aneurysm would have become stuck, increasing his risk of stroke, and he would have been forced to stay in the hospital and suffer from shortness of breath while being treated with ineffective medications. If Camzyos is covered by reimbursement, patients with similar difficulties will be able to receive many benefits and help. However, it seems that there are many difficulties in discussing reimbursement adequacy with previous treatment options as a comparison. In the real-world practice of medicine, many patients are unable to take their medications due to high non-reimbursement costs and are just waiting for reimbursement. -Camzyos is awaiting the DREC review. As mentioned before, medical personnel voice that there are no comparable treatments to Camzyos. What should government consider when determining the reimbursement? From a short-sighted point of view, reimbursement listing of a drug may seem like a disadvantage in health insurance finances, but if we expand our view on a macro level, we can see that this is not the case. If left untreated, patients with oHCM are at increased risk of developing heart failure, which inevitably increases the direct and indirect health care costs. In addition, an aneurysm increases the risk of stroke, which adds the cost of hospitalization to the cost of stroke medication. These costs can be considered in the long run as more patients are treated with Camzyos before complications occur. If their condition improves, the additional costs associated with emergency room admissions and treatment for other complications can be reduced. Ultimately, patients, doctors, and health authorities can benefit from each other.
- Company
- New oral psoriasis drug 'Sotyktu' available in hospitals
- by Eo, Yun-Ho May 14, 2024 05:48am
- BMS Korea’s Sotyktu (deucravacitinib). An oral psoriasis drug 'Sotyktu' is becoming available for prescription at general hospitals. According to industry sources, BMS Korea’s Sotyktu (deucravacitinib) has passed the drug committee (DC) of hospitals, including Seoul Asan Hospital, Severance Hospital, Konyang University Hospital, Soonchunghyang University Bucheon Hospital, Seoul National University Bundang Hospital, Soonchunghyang University Hospital Seoul, Ewha Womans University Medical Center, Chosun University Hospital, and Soonchunghyang University Cheonan Hospital. Sotyktu is expanding its prescription areas after being listed for insurance reimbursement in April. Sotyktu is the first TYK2 inhibitor approved for adults with moderate-to-severe plaque psoriasis. It has been 8 years since the approval in August of last year in South Korea for the treatment of adult patients with moderate-to-severe plaque psoriasis who are candidates for phototherapy or systemic therapy. It is now covered by health insurance. It is covered by insurance reimbursement for the treatment of patients over 18 with chronic severe plaque psoriasis who have symptoms lasting over six months. Coverage requirements include ▲Plaque psoriasis with over 10% of the total skin areas ▲Patients who have Psoriasis Area and Severity Index (PASI) score of over 10 ▲Patients who have undergone methotrexate or cyclosporine for over 3 months but cannot continue the treatment due to no response or side effects ▲Patients who have undergone light therapy or UPV photo therapy for over 3 months but cannot continue the treatment due to no response or side effects. Clinical efficacy of Sotyktu was demonstrated in Phase 3 POETYK PSO-1 and POETYK PSO-2 clinical studies, which compared the drug with placebo or Otezla in 1,684 adult patients aged 18 or over with plaque psoriasis. POETYK PSO-1 studies have shown that the Sotyktu treatment group had a PASI 75 response rate of 58.4% at week 16, which was significantly higher than the apremilast group’s 35.1% and the placebo group’s 12.7%. Furthermore, 53.6% of the Sotyktu treatment group achieved sPGA score of 0 or 1, higher than 32.1% in the apremilast group and 7.2% in the placebo group. In POETYK PSO-2 study, the Sotyktu treatment group had a PASI 75 response rate of 53.0% at week 16, which was significantly higher than the apremilast group’s 39.8% and the placebo group’s 9.4%. Furthermore, 49.5% of the Sotyktu treatment group achieved sPGA score of 0 or 1, higher than the apremilast group’s 33.9% and the placebo group’s 8.6%. Sotyktu’s high response was maintained up to 52 weeks. Choe Yong-beom, President of Korean Society for Psoriasis, said, "Previously, patients who did not respond to or have had side effects when treated with conventional treatments, such as systemic therapy or light therapy, had biological agents as their only option. Sotyktu, which offers the convenience of once-daily oral administration, is expected to meet the unmet needs of psoriasis patients.”