- LOGIN
- MemberShip
- 2025-12-22 17:41:10
- Company
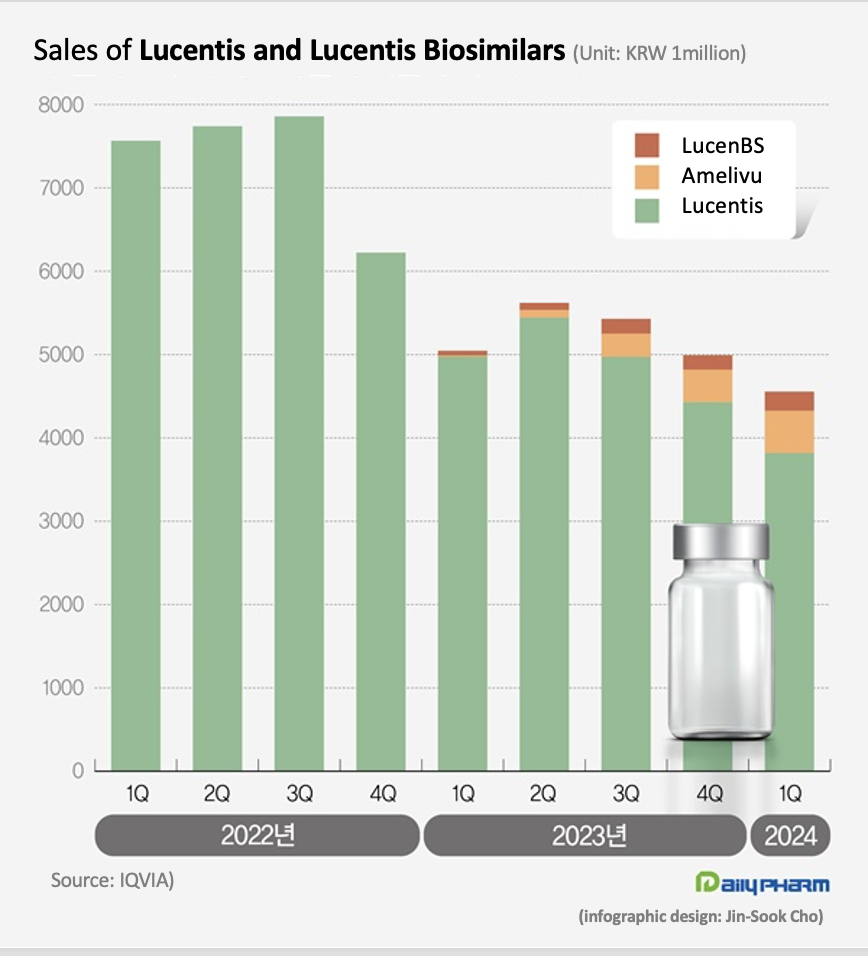
- Lucentis biosimilars occupy 16% of mkt in 1 year
- by Chon, Seung-Hyun Jun 19, 2024 05:46am
- The market size for the eye disease drug Lucentis has shrunk significantly. Last year, its biosimilars entered the market and reduced the drug price of Lucentis, reducing the market size. Although the market share of the two biosimilars remains a mere 16%, the price reduction of Lucentis saved more than KRW 10 billion in annual health insurance finances. According to drug research institution IQVIA, the market size of ranibizumab in Q1 was KRW 4.6 billion, down 9.7% YoY. Ranibizumab is the active ingredient contained in Lucentis. Lucentis, which is marketed by Roche and Novartis, is a drug used to treat eye diseases such as macular degeneration and diabetic macular edema. It is indicated for the treatment of ▲neovascular (wet) age-related macular degeneration, ▲diabetic macular edema, ▲proliferative diabetic retinopathy, ▲ vision impairment due to macular edema following retinal vein occlusion, ▲and vision impairment due to choroidal neovascularization. The ranibizumab recorded KRW 7.6 billion in sales in Q1 2022, down 33.3% from KRW 5 billion in Q1 last year. The market size for Lucentis shrank due to the drug price cut that followed the entry of its biosimilars. Samsung Bioepis and Chong Kun Dang received approvals for their Lucentis biosimilar products. Samsung Bioepis received approval for its Lucentis biosimilar Amelivu in May 2022, and Chong Kun Dang received approval for its LucenBS in October 2022. Amelivu and LucenBS have been reimbursed since January last year. The insurance price ceiling of Lucentis has been cut by 30% since February last year. The price of Lucentis 10mg (2.3mg/0.23mL) had fallen 30% from KRW 828,166 to KRW 579,716. The price of Lucentis Prefilled Syringe (KRW 826,231→KRW 578,362) also fell 30%. As a result, sales of Lucentis in Q1 were 3.8 billion won, down 23.3% YoY. Compared to Q1 2021, which was before the price cut, the company's sales have fallen by 49.5% in 2 years. While domestically developed biosimilar versions of Lucentis are working to accelerate market penetration, their current share in the market is less significant. In Q1, Amelivu sold KRW 500 million. Since its sales exceeded KRW 100 million in Q3 last year, its sales scale has gradually expanded, but its gap with the original drug is still large. Amelivu’s market share in Q1 was 11.1%. LucenBS’s Q1 sales were KRW 200 million, accounting for only 5.0% of the total market. The two biosimilars combined occupied 16.2% of the market, falling far behind Lucentis’s share. However, Amelivu and LucenBS’s supply at a relatively low price in the market is said to have significantly contributed to reducing the cost of medication for patients. The insurance ceiling price of Amelivu 10mg/ml is KRW 350,000, which is only at a 60.4% level of Lucentis’ price. Compared to the price of Lucentis before the price cut, it costs 42.3% of Lucentis’ previous price. The insurance price of LucenBS is KRW 150,000, which is 74.1% lower than Lucentis. This is more than 80% lower than the price of Lucentis before the biosimilar was launched. Initially, Chong Kun Dang listed LucenBS at an insurance price of KRW 300,000, then further reduced the price by 50% this year. In Q1, the Lucentis market shrank by 40.0% compared to 2 years ago. However, considering the drug price cut and the market entry of cheaper biosimilars, their usage has not decreased. The emergence of domestically developed biosimilars has resulted in annual health insurance savings of more than KRW 10 billion.
- Company
- Targeted therapies for HER2m NSCLC actively developed
- by Son, Hyung-Min Jun 18, 2024 05:49am
- The domestic and foreign pharmaceutical industry has taken up the challenge of developing a new drug for HER2-positive NSCLC. After Enhertu opened the door by obtaining the first marketing authorization as an anticancer drug targeting the HER2 mutation in Korea, many later entrants such as Yuhan Corp, Boehringer Ingelheim, and Hanmi Pharmaceutical have also started clinical trials. Until now, there have been a number of drugs targeting various gene mutations such as EGFR, ALK, ROS1, BRAF, MET, and RET in NSCLC, but none has targeted HER2. Approximately 2-4% of patients with NSCLC have a HER2 mutation. Due to its low prevalence, the companies had difficulty developing targeted therapies. This is why the clinical results of the latecomers are gaining interest. Boehringer Ingelheim disclosed early phase clinical trial results confirming the efficacy of its HER2-targeted antitumor candidate, and Yuhan Corp secured multinational IND approval for its HER2-mutation targeted antitumor drug candidate after Leclaza. Hanmi Pharmaceuticals also launched a new HER2-targeted NSCLC drug after failing with poziotinib. According to industry sources on the 18th, Boehringer Ingelheim's zongertinib (Development code name: BI-1810631) showed an effect in HER2-positive NSCLC. Zongertinib is a tyrosine kinase inhibitor (TKI) that binds to the HER2 mutation without inhibiting the wild-type EGFR. The Phase 1 trial, named BEAMION-Lung01, evaluated the efficacy of zongertinib in 36 patients with metastatic/advanced NSCLC who were refractory to standard of care and had HER2 mutations. The study was designed to determine if zongertinib can slow the progression of advanced NSCLC compared to the current standard of care, Keytruda plus platinum-based chemotherapy. The primary endpoint was set as an investigator-assessed overall response rate (ORR). Trial results as of July 31, 2023, showed that the ORR was 58% with zongertinib with a disease control rate (DCR) of 97%. In terms of safety, the most common treatment-related adverse events (TRAEs) were diarrhea and rash. Based on the tolerability and safety of the drug demonstrated in the Phase 1 trial, Boehringer Ingelheim plans to conduct a full efficacy evaluation of zongertinib through the Phase 2 trial. Korean companies including Hanmi and Yuhan also start developing treatments for HER2 NSCLC Yanghwa has also jumped into the development of HER2-targeted anticancer drugs. The company plans to develop a new drug for NSCLC to add on to its EGFR (HER1)-targeting anticancer drug Leclaza. The company’s candidate, YH42946, targets NSCLC patients with EGFR exon 20 mutations along with HER2 positivity. YH42946 was approved by the U.S. Food and Drug Administration (FDA) last month and was then granted to start a multinational Phase I/II clinical trial in Korea in the middle of this month. The company acquired the new anticancer drug candidate and its pipeline last year from a local biotech J Ints Bio. The Phase I/II trial initiated this time is the first trial evaluating the tolerability and safety of YH4294 in humans. In preclinical studies, YH42946 has shown anti-tumor effects against HER2 mutations and EGFR exon 20. It has also shown an effect on solid tumors such as breast and colorectal cancers. Hanmi Pharmaceutical is also out to develop new treatments after the failure of poziotinib. Its new anticancer pipeline, the ‘selective HER2 Exon20 insertion mutation inhibitor', which had not been disclosed until now, was found to have an anti-cancer effect based on its strong activity against HER2 exon20 insertion mutation and high selectivity for EGFR and has been confirmed to be a viable treatment for NSCLC. The company has a history of developing an oral treatment for HER2-mutant NSCLC. Hanmi Pharmaceutical and its U.S. partner Spectrum (now Assertio) developed poziotinib for locally advanced or metastatic NSCLC with HER2 exon 20 insertion mutations but ultimately failed to gain FDA approval. In 2022, the U.S. Oncologic Drugs Advisory Committee (ODAC) voted (9:4) that the benefits of poziotinib did not outweigh the risks, prior to the FDA's decision on whether to grant it marketing authorization. At the time, the ODAC noted that poziotinib lacks efficacy compared to Enhertu, which also targets HER2. Currently, the only targeted therapy option available for HER2-mutant NSCLC is Enhertu, developed by Daiichi Sankyo and AstraZeneca. On March 20, Enhertu’s indication was extended to the treatment of patients with unresectable or metastatic NSCLC whose tumors harbor an activating HER2 (ERBB2) mutation and who have received prior systemic therapy, including platinum-based chemotherapy. In the DESTINY-Lung02 study, Enhertu achieved a confirmed objective response rate (ORR) of 49%, complete response (CR) of 1%, and partial response (PR) of 48% as assessed by an independent centralized blinded review (BICR). Poziotinib achieved an ORR of 28% in the ZENITH20 cohort 2 study. Following the failure to receive approval, Spectrum deprioritized the development of poziotinib and reduced its R&D workforce by 75%. Therefore, it will be interesting to see if Hanmi Pharmaceutical’s newly developed HER2-targeted cancer drug will be able to go on further and receive approval.
- Company
- Eisai’s JAK inhibitor, 'Jyseleca,' has landed at hospitals
- by Eo, Yun-Ho Jun 18, 2024 05:48am
- Eisai Korea’s Eisai Korea’s 'Jyseleca,' a JAK inhibitor, is now available for prescription at general hospitals in South Korea. According to industry sources, Jyseleca, which is the fifth JAK inhibitor in South Korea, has passed the drug committee (DC) of Big 5 tertiary general hospitals, including Seoul National University, Seoul Asan Hospital, and Sinchon Severance Hospital, and national hospitals in major cities. It has expanded prescription areas after being listed for insurance reimbursement in November of last year. Jyseleca’s initial indication for reimbursement was for the treatment of rheumatoid arthritis and moderately to severely active ulcerative colitis. The reimbursement criteria are set for individuals who have had an inadequate response to conventional therapies or have no drug tolerance to each disease. For those who are over 65 years old, the criteria are set for individuals who have had an inadequate response to TNF-α inhibitors or have no drug tolerance. In July of last year, Jyseleca received conditional approval for reimbursement from the Drug Reimbursement Evaluation Committee (DREC) of the Health Insurance Review and Assessment Service (HIRA). It is indicated for the treatment of rheumatoid arthritis and ulcerative colitis. For the approval, Eisai accepted a price 90% below the actual average, exempted from the HIRA ceiling price negotiations, and quickly became reimbursable. In South Korea, JAK inhibitors, such as 'Xeljanz (tofacitinib),' 'Olumiant (baricitinib),' and 'Rinvoq (upadacitinib),' are being prescribed. It is to be watched whether Jyseleca would have a competitive advantage over these drugs. Since their launch, these drugs have been expanding indications and reimbursement criteria. Xeljanz additionally secured indications for ulcerative colitis and psoriatic arthritis, and latecomers, such as Rinvoq, are also expanding prescription areas in autoimmune diseases, including atopic dermatitis, Chron’s disease, and ankylosing spondylitis. Meanwhile, Jyseleca is a selective ATP-competitive and reversible JAK1 inhibitor. JAK1 transmits signals from a cytokine, and it is regarded as the key target for the treatment of rheumatoid arthritis. Recently launched treatments inhibit JAK2 or JAK3, depending on their mechanism. However, there are concerns that adverse reactions may occur, as two signaling pathways are involved in regulating immune cell proliferation and homeostasis. The FINCH1, FINCH2, and FINCH3 Phase 3 trials demonstrated the effectiveness of Jyseleca. In the FINCH1 trial, Jyseleca 200 mg treatment in patients with moderately to severely active rheumatoid arthritis reached ACR20 at 20 weeks more quickly despite continued treatment with MTX.
- Company
- Noh will retire from Amgen after 9 years since its inception
- by Eo, Yun-Ho Jun 17, 2024 05:46am
- Noh Sang-kyung, Amgen Korea general manager Noh Sang-kyung (61), general manger of Amgen Korea, who led Amgen’s Korean office since its inception is set to retire from the company. According to industry sources, Noh has recently confirmed his retirement from the company. He has served in this role for nine years since the company’s inception in South Korea in 2015. His successor has recently been appointed. Noh has been recognized for contributing to the stable establishment of Amgen in Korea and for delivering Amgen’s innovative products to Korean patients. Under Noh’s management, Amgen’s Korean office was able to list six launched products, including 'Prolia (denosumab),' 'Evenity (romosozumab),' 'Xgeva (denosumab),' 'Repatha (evolocumab),' 'BLINCYTO (blinatumomab),' and 'Kyprolis (carfilzomib),' in health insurance reimbursement listing. Meanwhile, Noh graduated from Sogang University with a degree from the Department of Life Sciences and worked at Lilly Korea, Roche Korea, and BMS. After working at Bayer Schering Pharma in 2007, he was appointed as the CEO of Bayer Schering Pharma Philippines office and the Head of Pharmaceuticals Business unit of Bayer Korea. In May 2015, Noh was appointed as the first general manager of Amgen’s affiliate.
- Company
- Elafibranor receives orphan drug designation in Korea
- by Eo, Yun-Ho Jun 17, 2024 05:46am
- The biliary cholangitis drug 'elafibranor' has been designated as an orphan drug in Korea. The Ministry of Food and Drug Safety (MFDS) recently announced the designation through an announcement. Elafibranor, a dual peroxisome-activated peroxisome receptor alpha/delta (PPAR α,δ) agonist that is being developed by Ipsen, received accelerated approval from the U.S. FDA for the primary biliary cholangitis indication on Nov. 10. More specifically, the drug is indicated for the treatment of adult patients with primary biliary cholangitis who have had an inadequate response to ursodeoxycholic acid (UDCA) or who cannot tolerate UDCA monotherapy due to tolerability issues. The FDA’s accelerated approval is based on data from the Phase III ELATIVE trial, which did not demonstrate improved survival or prevention of liver function decline. Ipsen is currently conducting the ELFIDENCE trial, a confirmatory clinical trial. The results of this study will determine whether the authorities will maintain the authorization. The ELATIVE trial demonstrated that elafibranor is an effective second-line treatment for patients with PBC with favorable benefit and risk data. Meanwhile, at the European Association for the Study of the Liver (EASL) 2024 Annual Congress, 2 additional analyses from the Phase 3 ELATIVE study, which evaluated the safety and efficacy of elafibranor in 161 primary biliary cholangitis patients with inadequate response or intolerance to ursodeoxycholic acid (UDCA), were presented one after another. The presented results were from Week 72 analysis, which showed that 30 of 108 patients (28%) in the elafibranor arm and 13 of 53 patients (25%) in the placebo arm remained on treatment through Week 72. Among these patients, 70% achieved biochemical response in the elafibranor arm, whereas no patients achieved biochemical response in the placebo arm.
- Company
- Pfizer immediately reapplies to extend reimb for Lorviqua
- by Eo, Yun-Ho Jun 14, 2024 05:47am
- Pfizer Korea has quickly reapplied for reimbursement of Lorviqua, its third-generation ALK anticancer drug whose reimbursement review process recently broke down at the drug pricing negotiation stage. According to Dailypharm’s coverage, Pfizer Korea submitted an application to expand insurance reimbursement for the ALK-positive non-small-cell lung cancer (NSCLC) treatment Lorviqua (lorlatinib) to the first-line treatment on the 12th. The drug's drug pricing negotiations with the National Health Insurance Service broke down in late May. It remains to be seen whether Lorviqua will be able to secure reimbursement in its second attempt and how quickly the discussions will move forward. In January, Pfizer submitted an application to convert the drug’s reimbursement status to general listing. At the time, its reimbursement expansion process was already underway, and given that Lorviqua has already undergone a pharmacoeconomic evaluation in the first-line setting and has completed the Health Insurance Review and Assessment Service review, the drug’s conversion to general reimbursement listing status is expected to be discussed in conjunction with the first-line reimbursement expansion Regardless of whether reimbursement is expanded or not, the pharmaceutical company’s will and the government’s flexible administration will be required to achieve a quick result. Lorviqua was specifically designed to penetrate the blood brain barrier (BBB). The drug’s high clinical value as a first-line treatment was recognized in the 5-year long-term follow-up results of the CROWN study that was presented at ASCO. Study results showed that Lorviqua reduced the risk of disease progression or death by 81% compared to crizotinib, with 60% of patients surviving without disease progression at 5 years. The risk of brain metastasis progression was reduced in 94% of patients, with only 4 of 114 Lorviqua-treated patients without brain metastases developing brain metastases. The reason the drug pricing negotiations broke down the last time is believed to be related to the ‘expenditure cap amount’ rather than 'drug price'. Lorviqua was granted pharmacoeconomic evaluation exemptions when it was first listed for reimbursement. PE exemption drugs are required to be reimbursed through the RSA Expenditure Cap type scheme. As such, a new cap amount would have been derived to account for the increased usage due to the expanded reimbursement during negotiations, which Pfizer was likely unable to accept. Press conference in front of the main gate of the National Assembly on the 13th
- Company
- Industry concern rises over the spread of the medical strike
- by Kim, Jin-Gu Jun 14, 2024 05:46am
- Some large hospitals are restricting access to pharmaceutical company employees’ access in the wake of a medical gap created by their resident doctors’ leave of absence. The pharmaceutical industry's situation worsens as the medical community is threatening to take a collective leave of absence due to conflict with the government over increasing medical school admissions. There are voices that say if the leave of absence, which is being made by doctors and some medical professors, spreads throughout the medical community, it will inevitably affect the industry’s Q2 performance results. There are already reports of companies predicting operating losses for their second quarter and some entering a state of emergency management. Medical representatives (MRs) in charge of general hospitals are on the verge of nervous breakdown, being unable to meet with medical professors. Pharma industry closely monitors the development of the medical strike ..." de facto emergency management system in place" According to industry sources on the 13th, the Korean Medical Association will take a collective leave of absence on the 18th. The association also announced a general strike at Yeouido Park in Seoul. In addition to the Big 5 hospitals, an increasing number of university hospitals have announced their participation in the strike. The faculty council of Seoul National University Hospital and Seoul National University Bundang Hospital decided to take a complete leave of absence on the 17th. Doctors at Severance Hospital will also take an indefinite leave of absence from the 27th. The Samsung Medical Center has also joined the collective leave of action, with other large hospitals also discussing plans. The medical strike, which had been ongoing amongst doctors and some medical school professors, seems to be spreading to large hospitals and clinics. The pharmaceutical industry is also watching the situation closely. Industry insiders say that if the medical community continues to take a leave of absence, the move will inevitably hurt the industry’s second-quarter earnings. Some companies are already forecasting losses for the second quarter and have effectively gone into emergency management. "Since last week, meetings involving executives from all departments have been held frequently," said an official from a large pharmaceutical company, "and it has been concluded that the company’s Q2 results will fall far short of the original target. We are currently discussing ways to minimize the damage." An official from another pharmaceutical company said, "If you look at our results through May, we're looking at a loss for the second quarter. The problem is that we don't have a good way to make up for it. We are keeping a close eye on the situation in the healthcare industry." An MR in charge of general hospitals confesses, "I haven't done anything for 2 months," The departments in charge of sales and marketing are also concerned about the spread of the medical strike. Departments that have launched or are planning to launch new products are said to be particularly affected by the prolonged medical-government conflict. An official from the marketing department of a domestic pharmaceutical company that recently launched a new product said, "Companies with existing products that have a high market share may not have a big problem, but companies that are planning to launch new drugs or new products are facing difficulties. We originally planned a large-scale launch symposium, but it has now been postponed indefinitely." The official added, "Recently, many medications are being prescribed in the long-term, for 6 months, making it very difficult for new drugs to be prescribed. This is a big risk for new drug sales and marketing teams." For departments related to general hospitals, the situation is worse as the MRs in charge could not meet with their medical professors since the start of the strike at the beginning of the year. Some surgeons and residents have returned to work, but in the internal medicine department, most full-time doctors and residents have not returned, with faculty members on call 2-3 times a week in rotation. An MR from a domestic pharmaceutical company in charge of a general hospital in Daejeon said, "I haven't worked since last month. Before that, I was at least receiving training, but now I am doing nothing. The company is just telling me to wait and see what happens. I don't want to force meetings with professors because it could backfire." An official in charge of clinical affairs at a multinational pharmaceutical company said, "It is also difficult to enroll patients in global late-stage clinical trials. Doctors are reluctant to attend even when we offer meetings to encourage participation in multicenter investigator-initiated trials (IITs). There are many restrictions to them attending not only investigator meetings but also pharmaceutical company events held in the evening or on weekends."
- Company
- The first APDS drug 'Joenja' gets orphan drug status in KOR
- by Eo, Yun-Ho Jun 13, 2024 05:48am
- Pharming Group A rare disease treatment 'Joenja' has received orphan drug designation in South Korea. The Ministry of Food and Drug Safety (MFDS) states this through the report on the designation of Orphan Drugs. Joenja (leniolisib), developed by the Dutch company Pharming group, is the first treatment for activated phosphoinositide 3-kinase delta syndrome (APDS). The drug was approved by the U.S. Food and Drug Administration (FDA) in March of last year. APDS is caused by a mutation in either the PIK3CD or PIK3R1 gene, essential to immune cell development and function. It occurs in 1 per 1-2 million. APDS patients commonly develop autoimmunity and inflammatory symptoms. They may suffer from ear, paranasal, and upper and lower respiratory infections. Moreover, APDS patients are susceptible to swollen lymph nodes and enlarged spleen, as well as an increased risk of cancer, such as lymphoma. Joenja’s approval was based on results from a multinational, triple-blind, placebo-controlled, randomized phase 2/3 clinical trial evaluating the efficacy and safety of the drug in 31 patients with APDS aged 12 years and older. Open label extension data of 38 patients receiving Joenja for an average of two years were also submitted. Randomized and placebo-controlled clinical results at 12 weeks demonstrated the clinical effects of Joenja 70 mg administered twice daily. It obtained a significant finding from the co-primary endpoints, which evaluated lymphoproliferation as measured by the reduction in lymph node size and increase in naïve B cells. It reflected the impact on immune dysregulation and normalization of immunophenotype. The change between Joenja and placebo for lymph node size was –0.25, and for the percentage of naïve B cells was 37.30. The most common adverse reactions were headache, sinusitis, and atopic dermatitis. Meanwhile, Pharming Group signed a license agreement with Novartis for Joenja in 2019. Novartis received US$10.5 million from Pharming Group when Joenja launched in the United States in April last year, as well as additional milestone and recurring royalty revenue.
- Company
- A growing trend of SC injections heats up competition
- by Son, Hyung-Min Jun 13, 2024 05:48am
- Pharmaceutical and biotech companies in Korea and worldwide focus on developing formulation modifications for anticancer agents. Following 'Tecentriq (atezolizumab)' SC (subcutaneous injection obtaining marketing authorization from the European Medicines Agency (EMA) in January, the R&D of 'Opdivo (nivolumab)' and 'Rybrevant (amivantamab)' is nearing the end. The industry attributes the recent booming development of SC formulations to their advantages over IV (intravenous) formulations, such as shortened administration time, improved convenience of administration, and reduced injection-related side-effects. According to the industry sources on June 11th, Bristol Myers Squibb (BMS)·ONO Pharmaceutical and Janssen disclosed the research outcomes of their SC formulations products, Opdivo and Rybrevant, respectively. Opdivo, an immunotherapy for cancerOpdivo, developed by BMS and ONO Pharmaceutical, is a PD-1-targeting immunotherapy for cancer. BMS conducted a clinical trial involving patients with renal cell carcinoma to change the Opdivo IV formulation to an SC formulation, and they obtained successful outcomes. The Phase 3 CheckMate-67T trial enrolled 495 patients with advanced or metastatic clear cell renal cell carcinoma (ccRCC) who have received prior therapy. For safety profile, the percentage of patients with topical adverse reactions of all grades in the Opdivo SC group was 8.1%, and in the IV group, it was 2.0%. Among the patients who were anti-drug antibodies (ADA)-positive, 15.2% of the patients in the SC group experienced mild topical adverse reactions of Grade 1-2, which resolved without the need of treatment. SC formulation of Rybrevant, a targeted therapy for cancer, has also shown effects in a clinical trial. Rybrevant, developed by Janssen, is a treatment for patients with non-small cell lung cancer (NSCLC) with EGFR exon 20 insertion mutations. A recently disclosed Phase 3 PALOMA-3 study demonstrated the non-inferiority of SC formulation Leclaza plus Rybrevant therapy to IV formulation Leclaza plus Rybrevant therapy. At a median follow-up of 7 months, SC formulation of Leclaza plus Rybrevant therapy was non-inferior to IV formulation Leclaza plus Rybrevant therapy. Yuhan SC formulation of Leclaza plus Rybrevant therapy had an ORR of 30.1% compared to 32.5% in IV formulation Leclaza plus Rybrevant therapy, meeting the standard for non-inferiority. For injection-related reactions (IRR), SC formulation of Leclaza plus Rybrevant therapy showed 13% IRR, which was significantly lower than 66% in IV formulation Leclaza plus Rybrevant therapy. The analysis suggests that improving Rybrevant’s administration will lead to greater synergy when combined with Leclaza. One of the concerns about combining oral Leclaza with IV formulation Rybrevant is that it may be less convenient due to the increased number of hospital visits. Since oral formulations for targeted therapy for lung cancer, such as Tagrisso (osimertinib) and Giotrif (afatinib), have been approved, Rybrevenat’s injectable formulation poses a weakness. Therefore, whether SC formulation of Rybrevant would be commercialized is attracting attention. Latecomers have started developing SC formulations Latecomers to developing anticancer agents are working to change formulations to reduce the administration time. Roche has succeeded in developing SC formulation of Tecentriq, an immunotherapy for cancer, and MSD is conducting a phase 3 clinical trial of SC formulation of Keytruda. Alteogen, which has a SC formulation modification technology, plans to develop technology for ADC SC platform. Alteogen expects the SC formulation of ADC to reduce adverse reactions. The company aims to launch the SC formulation of ADC into the market by 2028. The Korean pharmaceutical biotech industry is working on developing potential SC formulations of anticancer candidates. GI Innovation is developing a SC formulation of GI-102, a candidate immunotherapy for cancer. According to GI Innovation, the SC formulation of GI-102 has a bioavailability (BA) of up to 60% compared to an IV formulation. A clinical trial for the SC formulation of GI-102 will be conducted by confirming an appropriate dose in patients with various solid cancers. Once a proper dose of the SC formulation of GI-102 is determined, GI Innovation plans to conduct a clinical trial for an incremental dose in patients with solid cancer who have failed systemic therapies. Additionally, several companies are working on a new formulation modification other than IV to SC. Daehwa Pharmaceutical is developing an oral paclitaxel formulation, Liporaxel Sol. In a phase 3 clinical trial conducted by Daehwa Pharmaceutical’s partnering company in China, Haihe Biopharma, the efficacy and safety of Liporaxel have been confirmed to be non-inferior to paclitaxel injection.
- Company
- ‘Verify safety when using botulinum toxin for hair loss'
- by Nho, Byung Chul Jun 12, 2024 05:45am
- Dr. Jae-Hong Kim, Director of Planning and Policy at the Association of Korean Dermatologists (Chief Director, Yonsei Joeun Dermatology Clinic Gwangmyeong Branch)The market for hair loss treatment is growing day by day as hair loss, once considered a typical condition of middle-aged men, has become a general condition across all genders and ages. In particular, the market is being segmented across a wide age range and various lifestyles, and new treatments that complement the limitations of existing treatments are being actively developed. Dr. Jae-Hong Kim, Director of Planning and Policy at the Association of Korean Dermatologists, said, "Androgenetic alopecia (AGA), the most common type of hair loss, is generally treated with oral medications, but if the patient has inadequate drug response, the treatment’s effect decreases after a certain period of time. In recent years, procedures with different mechanisms of action, such as botulinum toxin, have emerged as a new alternative to treat hair loss." Botulinum toxin is a neurotoxin that inhibits the release of acetylcholine, a neurotransmitter that prevents muscles from contracting. It causes a temporary relaxation and shrinkage response in the muscle and is commonly used to treat wrinkles such as crow's feet and frown lines or to shrink overdeveloped muscles such as trapezius and calves. Kin explained, "When injected into the dermis, botulinum toxin has been shown to reduce the activity of TGF-ß1, which is responsible for hair loss. It is known to improve hair loss by increasing blood circulation by relaxing of the muscles around the scalp when injected intramuscularly." Research and publications on the effectiveness of botulinum toxin in treating hair loss are actively being conducted and published in the field. "There was a case of a man in his 30s and 40s who had advanced hair loss with thinning hair in the crown area along with scalp heat. He did not see any significant effect from oral medications, but experienced symptom improvement after receiving botulinum toxin injections in the balding areas.” However, as hair loss can have a significant impact on a patient's social and interpersonal life, it is important to carefully check the safety of the administered products. The safety and product history can be checked through the product’s FDA approval status and proven titer in the global market. In Korea’s botulinum toxin market, products such as Botulax have been approved by the FDA and have entered big markets such as Europe and China, and have been recognized for both its efficacy and stability. Accurate diagnosis and proper treatment by a medical professional are also important to achieve the best results. He added, "Patients tend to rely on arbitrary treatments or folk remedies, fearing side effects of oral medications or painful injections, which can cause them to miss the right timing to receive treatment. As various treatment methods are now available, patients should consult with a medical team to develop a treatment plan that takes into account each patient’s individual characteristics and the mechanism of action of the drug."