- LOGIN
- MemberShip
- 2026-03-09 20:48:32
- Policy
- "2030년까지 FDA 직접 허가 국산신약 사례 창출"
- by Lee, Jeong-Hwan Jan 19, 2026 09:02am
- [데일리팜=이정환 기자]보건복지부가 '제약바이오산업과'를 신설, 국내 제약산업 집중 육성 의지를 드러낸 가운데 임강섭 초대 과장은 "국산 신약이 자력으로 미국 식품의약국(FDA) 허가를 획득하는 사례를 만들겠다"는 포부를 내놨다.국내 제약사가 글로벌 빅파마에 신약 기술수출을 통해 미국, 유럽 등 해외 시장에 진출하는 현실을 뛰어 넘어 임상3상까지 오롯이 끝마친 뒤 블록버스터 신약으로서 직접 해외 허가를 따내는 원년으로 삼겠다는 의지다.18일 임강섭 과장은 복지부 전문기자협의회와 만나 "임상3상까지 끝낸 완제 신약을 개발해 FDA 허가까지 받아 직접 판매하는 사례를 5년 안에 만드는 게 목표고, 올해를 원년으로 삼아 2030년까지는 사례를 만들 것"이라고 피력했다.임 과장은 초대 제약산업과장으로 임명된 올해 무게중심을 둘 행정이 무엇이냐는 질문에 "임상3상 완료, FDA허가, 직접 판매에 성공하는 완제품 신약 개발"을 최우선 과제로 꼽았다.기술수출 지원도 지금보다 늘리겠지만, 우리나라 손으로 만든 신약이 빅파마 손을 빌리지 않고 해외 시장 문을 직접 두드릴 수 있게 돕는 복지부 지원에 방점을 찍겠다는 취지다.이를 위해 복지부는 최종 허가 가능성이 엿보이는 신약물질의 임상3상을 타깃으로 지원하는 전용 펀드를 운영한다.실제 복지부는 이를 위해 올해 예산안에 600억원 규모 정부 출자금을 편성한 상태로, 모태펀드 매칭을 합쳐 총 1500억원 규모의 '임상3상 특화 펀드'를 조성할 방침이다.임 과장은 "지난해 기술수출은 역대 최고치였는데 여전히 완제품 신약 해외 출시까지 가는 사례는 거의 없다"면서 "이젠 우리나라가 주체로서 임상3상까지 완료하고 FDA 승인을 받는 사례를 만들겠다"고 힘 줘 말했다.그러면서 "K-바이오 백신 펀드 집행 내역을 보면 전임상(동물실험)에 약 50%, 임상1상~2상에 약 50%가 쓰였는데 임상3상 투자 사례가 없어 아쉽다"며 "임상3상 투자 공백을 어떻게 메울지 방법을 고민했고, 올해 예산을 토대로 임상3상 특화펀드를 만들 것"이라고 설명했다.또 "국민성장펀드 150조원 중 제약·바이오·백신 분야에 11조6000억원을 5년간 활용한다고 하니, 이 자금을 후기 임상 투자로 연결하는 구상도 하고 있다"며 "제약바이오 기업들이 후기 임상 단계에서도 펀딩을 받을 수 있다는 신호를 전달해 임상·사업 계획을 세울 수 있게 하겠다"고 부연했다.임 과장은 복지부의 기술수출 지원 역시 계속 지원할 계획이라고 했다.제약바이오 벤처·스타트업 기업이 기술수출을 기점으로 중소기업, 중견기업으로 성장하고 또 더 큰 단계로 활로를 모색하는 경로를 복지부가 돕겠다는 얘기다.복지부는 글로벌 오픈이노베이션 예산으로 104억원을 편성한 상태로 중소기업벤처부 예산과 합쳐 규모를 더 키울 수 있도록 협업 방안을 만들고 있다.아울러 복지부는 중기부와 협력해 상반기 중 제약바이오 벤처와 협의체를 만들어 의견을 수렴하고 규제 완화 요구안과 지원안 등을 정리하는 작업도 추진한다. 이를 근거로 하반기부터 규제 선진화 작업과 내년도 예산 작업에 나선다는 게 임 과장 계획이다.임 과장은 "글로벌 오픈이노베이션 예산으로 지원하게 될 대상 기업 수는 32개사 정도다. 플랫폼 기술개발 R&D의 경우 올해 기획해서 내년 예산에 담는 방안을 검토 중"이라며 "기존 R&D가 주로 후보물질-전임상-임상에 집중됐다면 앞으로는 플랫폼 기술 자체에 대한 투자도 지원하려 한다"고 말했다.이어 "제약바이오 산업과란 명칭으로 전담과가 새로 생긴 건 상징적 의미가 크다. 책임감을 느낀다"며 "지난해 9월 발표된 제약바이오 대도약 전략의 후속 예산과 행정 조치가 올해부터 본격적으로 실행된다. 산업계에 변화된 모습을 제시할 것이고 첫 메세지가 혁신형 제약사 인증제 개편안 발표가 될 것"이라고 덧붙였다.
- Policy
- Pharma-MOHW disagree on drug pricing policy
- by Lee, Jeong-Hwan Jan 16, 2026 08:52am
- The Korean pharmaceutical industry has announced its stance that the government's drug price reform plan, which centers on generic price cuts and preferential pricing for "Innovative Pharmaceutical Companies," is too vague and requires both a delay in implementation and significant revisions.The industry requested a sufficient grace period, arguing that the plan excludes long-term impact assessments on the pharmaceutical-bio industry and public health, which could lead to industrial contraction.In contrast, the government stated it has no intention of delaying its plan to submit and finalize the reform at next month's (February) Health Insurance Policy Deliberation Committee for full implementation in July. This has left the gap between the two sides unbridged.As the pharmaceutical industry and the government have not bridged the divide in opinion, the conflict over drug pricing reform is expected to continue.On the 14th, the "National Assembly Forum on Drug Price Policies for Becoming a New Drug Powerhouse," hosted by Democratic Party Representatives Lee Un-ju, Seo Young-seok, and Kim Yoon, brought together the MOHW and industry representatives to exchange views.Pharmaceutical Industry, "Concerns over job losses, supply instability, and slowed growth"Hong Jeong-Ki, Managing Director of the Korea Pharmaceutical and Bio-Pharma Manufacturers Association (KPBMA), pointed out that repeated drug price cuts, executed 10 times since 1999, have gradually shrunkne the industry's overall profitability and investment capacity.Hong also expressed concerns that large-scale cuts to generic prices, as foreshadowed by the MOHW, would inevitably deepen reliance on low-cost imported raw materials and finished imported drugs.While price cuts might yield short-term fiscal savings, Hong warned that, based on implementation of policies in the past, they could lead to long-term employment shrinkage, instability in the supply of essential medicines, and a slowdown in industrial growth.Hong argued that the implementation of price cuts should be reconsidered after a comprehensive assessment of the impact on the industry and public health. Hong argues the need for a sufficient grace period for a successful landing and the establishment of a government-industry governance system.Furthermore, he requested fair compensation for companies that contribute to innovation and supply stability.The compensation aims to provide uniform preferential pricing for all Innovative Pharmaceutical Companies and to grant price benefits to non-certified companies that demonstrate excellent R&D performance and facility investment."The current reform plan is structured such that damages from strengthened regulations increase while compensation for innovation and supply stability remains limited," Hong pointed out. "This could act as a barrier to becoming one of the world's top five pharmaceutical powerhouses."He emphasized, "The implementation of drug price cuts must be reconsidered after an assessment of both short- and long-term impacts. We request a sufficient grace period to ensure a soft landing for the system," adding, "It is also necessary to establish a government-industry governance structure for constructive discussions on drug price policy."He further stressed, "By specifying the innovation-based price addition period and ensuring fair value compensation, we must build a virtuous cycle that accelerates innovation, such as through R&D reinvestment, and leads to the creation of global new drugs," and maintained, "Cuts to the prices of existing listed generics should be minimized to secure investment capacity and ensure supply stability.""Preferential pricing plan fails to reflect company contribution and diversity"Kim Sang Jong, Managing Director of Hanmi Pharmaceutical, also criticized the reform, stating it could weaken the foundation for domestic companies to continue investing in new drugs.Kim argued that cutting existing generic prices without accounting for a company's contribution to new drug R&D creates a structure in which larger companies with higher investment volumes incur greater absolute losses. In his view, the policy is becoming an overall price cut premised on the notion that Korean generics are expensive compared to overseas markets, rather than a system that guarantees fair returns on investment.Notably, Kim highlighted that limiting preferential pricing to "Innovative Pharmaceutical Companies" and a few others fails to benefit the many firms that contribute to the industry through Phase 2/3 trial investments, manufacturing facility expansions, quality advancements, and employment of research personnel.Kim added that extending the price increase period for Innovative Pharmaceutical Companies by three years is only effective in the early stages of the patent-expired market. Once generic market share expands in the later stages, prices drop significantly, making the compensation effect felt by companies very limited."The resources needed for new drug R&D, maintaining production facilities for stable supply, and maintaining employment currently come from generic sales," Kim criticized. "It is questionable whether an overall price-cutting policy aligns with reward investment."Kim further criticized, "The financial resources required for new drug R&D, maintaining production facilities for stable supply, and sustaining employment come from the sales of existing listed generics. For now, this is unavoidable," adding, "While the MOHW stated it would focus on outcomes such as innovation, I question whether implementing a blanket price-cutting policy, based on the idea that high generic prices can now be lowered, actually aligns with the policy goal of rewarding investment."He added, "It is questionable whether it is truly necessary to further divide preferential drug pricing among Innovative Pharmaceutical Companies into upper and lower tiers. Once the three-year preferential period ends, generic expenditures drop significantly. We are not sure if this can be considered a reward for Innovative Pharmaceutical Companies," adding, "Pharmaceutical companies also need to analyze the reorganization of the drug price system. Since the announcement on November 28 last year, time has been needed to run simulations to determine how to manage operations in line with the policy goals. A grace period (for the drug price cuts) is necessary."MOHW "Current system has reached its limit... will reform to minimize industry concerns"Despite such requests from Hong and Kim to delay implementation and revise the preferential pricing policy, the MOHW rejected these requests, stating that the current system has reached its limit and cannot be further delayed.Kim Yeon-Sook, Head of the Division of Pharmaceutical Benefits at the MOHW, explained, "This reform is being implemented because the current system has reached its limit. It is a structural policy change designed to improve these limits, strengthen the supply stability of both new and essential drugs, and maintain the sustainability of national health insurance," and explained, "Other countries also periodically adjust generic prices while considering fiscal efficiency.""While our drug price system has previously lacked predictability and fallen short of achieving the goal of generic activation, this reform is different," Kim added. "Unlike past approaches focused solely on reducing drug expenditures, this reform includes mechanism to reward innovation based on investment, even for generics."Kim concluded, "The government views this reform as a golden time for structural improvement and a leap forward for the industry, centered on Innovative Pharmaceutical Companies. We will implement a drug price system that leads to rewards for the industry to minimize the concerns of domestic pharmaceutical firms."
- Policy
- ‘Drug innovation is concentrated around specific diseases’
- by Jung, Heung-Jun Jan 16, 2026 08:52am
- The OECD has raised concerns that pharmaceutical innovation is increasingly concentrated in specific disease areas, while placing healthcare financial sustainability at the center of policy debate.It has stressed that the price increases of new drugs must be reviewed to determine if they actually lead to meaningful health gains, and that a comprehensive evaluation is necessary when assessing access to medicines.On the 15th, the Health Insurance Review and Assessment Service (HIRA) announced the results of its research on the Revised OECD Health System Performance Assessment framework indicators.The OECD HSPA framework is designed to compare how effectively national healthcare systems are functioning across countries.According to HIRA’s analysis, pharmaceuticals and medical technologies were newly positioned as core foundational elements determining system performance in the 2024 revised OECD HSPA framework.The revision reflects lessons from the COVID-19 pandemic, during which vulnerabilities in global supply chains for essential medicines and medical devices, as well as shortages in intensive care capacity and healthcare infrastructure, were exposed.Sustainability perspectives were particularly strengthened. The HIRA research team explained, “(The revised framework) critically questions whether rising prices of new drugs translate into actual improvements in health value and addresses the sustainability of health finances as a key policy challenge.”They added, “The OECD also points out the imbalance created by innovation being concentrated in specific disease areas, and calls for a fundamental review of the pharmaceutical incentive structure and the rationality of current pricing models.”Changes were also made to concretize the concept of drug accessibility. This signifies that accessibility should be evaluated as a multidimensional, people-centered concept, not merely based on a product's availability.The research team stated, " The revised framework defines access to medicines as a multidimensional concept encompassing availability, affordability, geographic accessibility, acceptability, and quality. This implies the need for a comprehensive assessment of barriers across the entire medicine lifecycle, including regulatory approval, health technology assessment, reimbursement listing, and prescribing and dispensing practices.”They further noted, ”The OECD has published a report that serves as a foundation for developing indicators to monitor such complex dimensions of access."
- Policy
- MOHW·KHIDI to focus on fostering innovative pharma companies
- by Lee, Jeong-Hwan Jan 16, 2026 08:52am
- The Ministry of Health and Welfare (MOHW) and the Korea Health Industry Development Institute (KHIDI) have set fostering of 'Innovative Pharmaceutical Companies' as a primary focus for the New Year. As the executive government bodies overseeing Innovative Pharmaceutical Companies, the MOHW and KHIDI plan to fully utilize the certification system for Innovative Pharmaceutical Companies as a core engine for the global expansion of the domestic pharmaceutical and bio-industry.These are the details from the New Year's policy directions announced by the MOHW Pharmaceutical and Bio-Pharma Industry Division on the 15th, as well as the major business report from KHIDI.Currently, the Innovative Pharmaceutical Company certification system is facing a shift. The core of the government's drug price system reform plan, which it announced will be implemented this year, involves significantly differentiating drug price preference rates based on whether a company holds the Innovative Pharmaceutical Company certification. The MOHW is preparing to improve the certification system to address controversies over its practical effectiveness.In this context, the MOHW and KHIDI have expressed their determination to leverage innovative pharmaceutical companies to advance the domestic pharmaceutical industry.First, Yim Kang-seop, Head of the Division of Health Industry Policy at the MOHW, plans to lead the structural improvement of the domestic industry through the reorganization of the certification system.Since the certification status is linked to the drug price reform plan, which determines the preferential price ratios for pharmaceutical companies, the reorganization aims to provide companies with specific signals regarding innovation.Yim explained, "Over the past 10 years, innovative pharmaceutical companies have contributed to some extent in shifting the domestic industry ecosystem toward a focus on new drugs. However, even among the 48 certified companies, a significant number still rely on generics, accounting for more than 40% of their sales. We must objectively acknowledge that some companies are becoming complacent with generics."Yim further emphasized, "We will strengthen systems so that innovative pharmaceutical companies can drive changes across the entire industrial ecosystem. We are engaged in sophisticated, strategic deliberation to ensure the drug pricing system functions as a mechanism that drives innovation, new drug development, and global expansion. We will enhance performance through the certification system reform."KHIDI also stated its goal of actively fostering the pharmaceutical industry to realize the Lee Jae Myung administration's national task of becoming one of the global top 5 pharmaceutical and bio-health powerhouses.First, KHIDI will support the National Bio-Innovation Committee, a governmental governance body, to provide more professional and systematic support for bio-health policies, and will establish statistics-based policies to respond to AI and new technology environments.Additionally, KHIDI will take full responsibility for managing the expanded healthcare R&D budget of KRW 1.2 trillion.KHIDI plans to focus on strengthening industry competitiveness by identifying policies that systematically cultivate innovative pharmaceutical companies.Hong Heon-Woo, KHIDI's Director of Planning, announced, "We will systematically foster innovative pharmaceutical companies and pursue global expansion through linked support from AI-based advanced medical device R&D to commercialization," adding, "KHIDI's plan includes striving to achieve KRW 2 trillion in healthcare R&D by 2030 and promoting strategic R&D investment centered on promising technologies by discovering representative technologies that citizens can experience."
- Policy
- Minister Jeong "Regional·essential healthcare doctor system"
- by Lee, Jeong-Hwan Jan 16, 2026 08:52am
- Minister of Health and Welfare Jeong Eun KyeongMinister of Health and Welfare Jeong Eun Kyeong stated, "The results of the Medical Personnel Supply and Demand Estimation Committee, while subject to realistic constraints, are the best possible outcome based on currently predictable data and a consensus-driven process."The Ministry of Health and Welfare (MOHW) reaffirmed its position that the newly increased medical workforce will be dedicated exclusively to personnel working in regional and essential healthcare sectors.Minister Jeong made these remarks during the 3rd meeting of the Health and Medical Policy Deliberation Committee held at the International Electronics Center in Seocho-gu, Seoul, at 4 p.m. on the 13th.The meeting discussed detailed plans for the deliberation criteria for the scale of medical personnel training from 2027, following the 1st meeting held on December 29 last year.First, the committee reconfirmed its agreement for the estimation results of the Medical Personnel Supply and Demand Estimation Committee (the Estimation Committee). The Estimation Committee, composed of a majority of members recommended by provider organizations, held 12 rounds of discussions.The meeting discussed the 1st deliberation criterion from the 1st meeting, focusing on resolving regional healthcare disparities and shortages of essential and public medical personnel. Specifically, the committee discussed a plan to apply the increase in medical personnel quota after 2027 to the quota for the regional doctor system.Furthermore, it was decided to consider the scale and timing of graduates resulting from the establishment of a "Public Medical Training School" (tentative name) and the creation of new medical schools in regions currently without them.The committee will also consider the second and third deliberation criteria, detailing future changes in the medical environment and healthcare policy shifts, by including all combinations of the three demand models and two supply models adopted by the Estimation Committee.Regarding the fourth criterion, related to ensuring the quality of medical education, the committee explored ways to keep the fluctuation rate of the 2027 admission quota within a certain level compared to the 2026 recruitment (3,058 students total) and to ensure that small-scale medical schools can secure an appropriate number of students for education.The committee also decided to consider the reality that students from the 2024 and 2025 classes are currently attending lectures together.For the final criterion, related to ensuring predictability and stability, the committee discussed applying the quota based on the 2025 estimate for five years from 2027 to 2031, in line with the mandatory five-year cycle for supply and demand estimation.Given that students entering during this period will graduate over five years from 2033 to 2037, 2037 was set as the base year for supply and demand management. The plan for the next estimation, to be conducted in 2029, was reviewed, taking into account the timing of the next quota application (the 2032 academic year) and the advance notice system for university admissions.The Health and Medical Policy Deliberation Committee plans to incorporate the results of these discussions on the application of deliberation criteria and submit training scale proposals for multiple scenarios at the next meeting.Minister Jeong stated, "We plan to review the scale of the medical workforce after 2027 based on the estimation results. In today's meeting," adding, "we will discuss agendas to detail the deliberation criteria applied to the various estimation results derived from the demand and supply models presented by the Estimation Committee."Minister Jeong added, "The most important principle is that the ultimate goal of discussing the scale of the medical workforce is to strengthen regional, essential, and public healthcare, which is currently in crisis," and stressed that, "We must also consider upcoming policy changes, such as the legislation for training and supporting regional doctors and the special law for strengthening essential healthcare and resolving regional medical gaps, which is currently awaiting passage in the National Assembly."Minister Jeong concluded, "We have previously failed to apply crucial deliberation criteria such as the qualitative level of medical education, the situation at educational sites, and sufficient predictability for those sites to train high-quality medical personnel," adding, "Through thorough discussion in today's meeting, we plan to refine further the review criteria presented in the first meeting."
- Policy
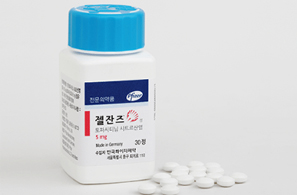
- Companies do not renew Xeljanz generic licenses
- by Lee, Tak-Sun Jan 15, 2026 08:47am
- Pfizer’s rheumatoid arthritis drug Xeljanz TabDespite the five year await for Xeljanz’s patent expiry, an increasing number of companies are abandoning sales of the generic versions of their rheumatoid arthritis treatment ‘Xeljanz.’This is largely attributed to drastic changes in the competitive landscape compared to the time of development. Some in the industry point to the negative consequences of so-called “blind approvals,” where companies follow competitors without question.According to the Ministry of Food and Drug Safety (MFDS) on the 14th, the license for Youngil Pharm’s Youngil Tofacitinib Tab 5 mg (tofacitinib aspartate) expired on the 14th and was removed from the product registry. This marks the 29th tofacitinib product to expire without renewal.By March, an additional six products are scheduled to expire, suggesting that more companies may forgo renewal.Of the 45 products that remain approved, only 14 have been listed for reimbursement.This situation reflects how the market environment changed dramatically during the 5-year wait for the original product’s substance patent expiry.Generic manufacturers attempted to bypass the extended substance patent by developing salt variants. Had this strategy succeeded, launch would have been possible after November 23, 2020, instead of waiting until the original substance patent expired on November 23, 2025.However, after legal disputes, the Supreme Court ruled in favor of the originator, Pfizer. The latecomers who received approval in 2020 had to wait until the substance patent expiry date of November 23, 2025.As a result, companies spent five years unable to launch, merely waiting for patent expiry. When the patent finally expired in November last year, several companies launched their products.However, the market had changed substantially compared to five years earlier. While Xeljanz became a blockbuster product generating sales in the KRW 10 billion range due to expanded reimbursement, its growth faced limitations as strong competitors emerged.New JAK inhibitors like Lilly's Olumiant and AbbVie's Rinvoq have entered the market since then. According to 2024 UBIST outpatient prescription data, Xeljanz recorded KRW 14.3 billion, Olumiant KRW 17.1 billion, and Rinvoq KRW 26.1 billion, showing Olumiant and Rinvoq surpassed Xeljanz in terms of sales.Furthermore, to counter latecomers, Pfizer released a more convenient extended-release formulation for Xeljanz, making it even harder for competitors to challenge it. The extended-release formulation, Xeljanz XR Tab 11mg, also holds a patent valid until March 12, 2035.As a result, more generic manufacturers are choosing not to renew their approvals despite the original drug’s substance patent expiry.Some point out that this incident highlights one of the drawbacks of the ‘blind approval’ system for latecomers. Many domestic pharmaceutical companies operating primarily in generics tend to develop products simply in line with the original drug’s patent expirations.Moreover, under Korea’s patent-linkage system, the first generic applicants that successfully bypass patents are granted nine months of market exclusivity. This has triggered a rush of follow-the-leader development, where competitors simply follow suit once a rival starts developing a generic.An industry executive commented, “In the case of some Xeljanz generics, the market has certainly changed, but fundamentally, the problem lies in companies blindly following competitors instead of pursuing independent business strategies, which has now backfired.”
- Policy
- 3-month import suspension for PCV 'Prevenar 20'
- by Lee, Tak-Sun Jan 14, 2026 09:32am
- Product photo of 'Prevenar 20 Pre-filled Syringe' The imported pneumococcal vaccine 'Prevenar 20 Pre-filled Syringe' will be subjected to a three-month suspension of import operations starting on the 12th. However, no issues are expected regarding its supply.This is interpreted as having sufficient existing imported inventory. As this vaccine is the most in-demand in the pneumococcal vaccination market, concerns about supply instability arose following the import suspension.The Ministry of Food and Drug Safety (MFDS) announced that the import operations for Prevenar 20 Pre-filled Syringe (Pneumococcal 20-valent Conjugate Vaccine [Diphtheria CRM197 Protein]) will be suspended for three months, from the 12th of this month to April 11th.The administrative measure was issued in accordance with relevant laws after it was confirmed in June last year that needles of a different specification than the authorized ones were being used.At that time, the MFDS distributed a safety letter ordering a temporary suspension of use. Pfizer faced significant challenges just as it launched a new product.However, after the issue was resolved, the product was initially supplied to the market through co-promotion with Chong Kun Dang. In October of that year, the supply was expanded as it was selected for the National Immunization Program (NIP) for infants from 2 months to children under 5 years of age. Once chosen for the NIP, eligible recipients can receive the vaccination free of charge.Concerns arose following the news of the sudden import suspension, but it has been confirmed that there are no issues with the supply.The MFDS stated, "Currently, there are no cases reported to the MFDS regarding the supply of this item," and added, "It has been confirmed by the company that there are no supply-related issues."Because this vaccine is used in the NIP and adult vaccinations are increasing, it seems that sufficient inventory was secured before the suspension. However, some question the effectiveness of the administrative measure, noting that if inventory is adequate, a fine should have been imposed rather than an import suspension.Prevenar 20 is the first new pneumococcal vaccine introduced by Pfizer in 14 years, adding seven serotypes (8, 10A, 11A, 12F, 15B, 22F, and 33F) to the previously available Prevenar 13.This vaccine is expected to replace the existing Prevenar 13. While competing products include Prodiax 23 and Vaxneuvance, Prevenar 20 is anticipated to take No.1 in the market, given that the previous Prevenar 13 has dominated with an outstanding market share.
- Policy
- Keytruda reimb extended… estimated claims of KRW 2 trillion
- by Jung, Heung-Jun Jan 14, 2026 09:31am
- While coverage has been strengthened with the expansion of reimbursement this month of the immuno-oncology drug Keytruda (pembrolizumab), the reimbursement expansion is expected to generate a record-high projected claims amount of KRW 238.4 billion, lighting warning signals for the management of Korea’s National Health Insurance finances.At the end of last year, the Ministry of Health and Welfare announced estimates that approximately KRW 1 trillion would be saved through its drug pricing system reform.However, when viewed in terms of net financial impact, the increase in claims for this single drug now accounts for roughly 20–25% of the savings generated from price cuts on listed generics.This is why calls are growing for stronger post-listing control, as more multi-indication blockbuster drugs enter Korea’s reimbursement system.According to industry sources on the 13th, the estimated reimbursement claims for MSD Korea’s immuno-oncology drug Keytruda represent the largest amount ever recorded, exceeding the KRW 2.2 trillion recorded for Paxlovid.This reflects the significant impact the drug is expected to have on national health insurance finances. Previously, Keytruda had been reimbursed for four cancer types, including non-small cell lung cancer. However, starting this year, reimbursement has been expanded to cover 17 regimens across nine cancer types, including head and neck cancer.The number of patients covered is expected to gradually increase from 3,258 to 6,680. Accordingly, the projected claim amount is expected to rise from KRW 178.8 billion in the first year to KRW 238.4 billion.In 2022, Keytruda successfully expanded coverage for first-line treatment of non-small cell lung cancer, among other indications. At that time, the Ministry of Health and Welfare estimated annual claims of KRW 1.762 trillion.Since then, Keytruda, which already generates annual sales exceeding KRW 400 billion as a single product, has continued to expand its reimbursement coverage, growing its market presence.The fact that coverage, previously concentrated on NSCLC, now extends to various cancer types, including female cancers, is welcome news for patients. However, the burden on health insurance finances is inevitably growing.The NHIS also states it will strengthen post-monitoring in consideration of the financial impact. As similar cases are expected to increase gradually, it is also contemplating enhanced post-approval management measures.An NHIS official said, “For new drugs with expanded reimbursement coverage, post-management is conducted through the price-volume linkage system. While other institutions may also have financial management measures in place, NHIS is considering additional post-management strategies as well.”
- Policy
- MFDS approves Servier’s Voranigo Tab in KOR
- by Lee, Tak-Sun Jan 14, 2026 09:31am
- The Ministry of Food and Drug Safety (MFDS, Minister Yu-kyoung Oh) announced on the 13th that it has approved Servier’s imported orphan drug ‘Voranigo Tab (vorasidenib) 10mg·40mg.’This drug is indicated for the treatment of Grade 2 astrocytoma or oligodendroglioma that have a susceptible isocitrate dehydrogenase-1 (IDH1) or isocitrate dehydrogenase-2 (IDH2) gene mutation in pediatric and adult patients aged 12 years and older weighing at least 40 kg, following surgery, including biopsy, major resection, or complete resection.A biopsy refers to the procedure in which a portion of tissue or cells is collected and examined under a microscope for diagnostic purposes.IDH (Isocitrate Dehydrogenase) mutations cause the abnormal production of excessive metabolic substances (2-HG), which promote the growth and survival of cancer cells.Astrocytoma and oligodendroglioma are types of gliomas, tumors arising from glial cells in the brain and spinal cord. Gliomas are a major category of brain tumors.Voranigo is an IDH-targeted therapy that inhibits mutated IDH1 and IDH2, thereby reducing the production of the carcinogenic substance 2-hydroxyglutarate (2-HG) and suppressing tumor cell proliferation. The Ministry of Food and Drug Safety expects the new approval to provide a new treatment opportunity for patients with brain tumors that are positive for IDH1 or IDH2 mutations.
- Policy
- Reimb re-evaluation active ingredient results to be unveiled
- by Jung, Heung-Jun Jan 14, 2026 09:31am
- The announcement of active ingredients subject to this year's reimbursement re-evaluation is expected to change slightly from the previously mentioned seven ingredients. This is due to changes in the re-evaluation selection criteria.According to industry sources on the 14th, an agenda regarding the 2026 reimbursement re-evaluation is scheduled to be tabled at tomorrow's Drug Benefit Evaluation Committee (DBEC) meeting.Furthermore, the re-evaluation selection criteria are expected to be discussed. The criteria regarding the number of countries where the drug is listed and the scale of the claimed amount will be removed. Ingredients that need re-evaluation of their clinical usefulness are expected to be included.When the government announced the drug price system reform plan last November, it pre-announced a restructuring of the criteria to include: ▲ active ingredients for which health authorities in A8 countries have initiated clinical or reimbursement adequacy re-evaluations ▲ cases where data or clinical evidence conflicting with previously reported efficacy has been published ▲ medications for which the necessity of re-evaluation has been suggested by academic societies and experts.This means that instead of classifying by listing countries or claim scale as in the past, active ingredients assessed to require a review of clinical usefulness will be designated for re-evaluation.The Health Insurance Review and Assessment Service (HIRA) is expected to listen to the opinions of institutions and academic societies and further strengthen its monitoring role regarding changes in claiming trends.If the reimbursement re-evaluation criteria are revised after passing through the DBEC and the Health Insurance Policy Review Committee, the preparation of additional procedures, such as an expert advisory committee for reviewing ingredient designation, is also anticipated.Additionally, the results of the reimbursement re-evaluation will be simplified to either "reimbursement exclusion" or "selective reimbursement." This means that the breakthrough of maintaining reimbursement through voluntary drug price cuts by pharmaceutical companies will no longer exist.If clinical adequacy cannot be proven, it is expected to lead to either an exit from insurance coverage or a change in patient co-payments.The seven ingredients discussed as targets for this year's re-evaluation at last year's HIPDC subcommittee were Ginkgo biloba leaf dried extract, dobesilate calcium hydrate, kallidinogenase, meglumine gadoterate, diacerein, Afloqualone, and octylonium bromide.Because the reimbursement re-evaluation criteria are changing, some fluctuations are expected in the seven ingredients selected based on the previous criteria. Meanwhile, this DBEC meeting is the first since the replacement of the 10th-term members. The committee has launched with 74 members, including Professor Seung Hyuk Choi of Samsung Medical Center, Professor Kim Seong-hwan of Seoul St. Mary's Hospital, Professor Seh-Hyon Song of Kyungsung University College of Pharmacy, and Professor Hyunah Kim of Sookmyung Women's University College of Pharmacy.