- LOGIN
- MemberShip
- 2025-12-24 12:42:40
- Policy
- Closing the opinion gap important for the generic '1+3 bill'
- by Lee, Jeong-Hwan Jun 21, 2021 05:51am
- With the bill limiting the participating generic makers to three consignees per consignor when conducting joint biological equivalence tests or clinical tests awaiting review by the National Assembly’s Legislation and Judiciary Committee., whether the opinion gap between the large pharmaceutical companies and small- and medium-sized pharmaceutical companies can be closed is gaining attention. Despite the unanimous decision made by the Health and Welfare Committee, concerns have been rising on the influence the divided positions held by different sized pharmaceutical companies, which, if not resolved, may have on the process and result of the review conducted by the Legislation and Judiciary Committee. On the 20th, the pharmaceutical industry has been fighting a psychological battle behind the scenes on the legislation of the ‘1+3 restriction of generics’ bill The Health and Welfare Committee that passed the bill agreed that the 1+3 bill will reduce the excessive amount of generics and drugs requiring data submission and significantly improve the structure of the pharmaceutical drugs in Korea, however, the pros and cons are still fiercely being judged in the industry. While the large pharmaceutical companies that own the capability and economic scale to develop new drugs are strongly in favor of the 1+3 bill, the small- and mid-sized pharmaceutical companies that profit from generics and data submission drugs have been complaining that the bill will serve as leverage to promote the collapse and job cuts in their companies. In particular, the small- and mid-sized pharmaceutical companies have also criticized that, regardless of its purpose, legislating a bill to reduce the excessive number of items is out of order as they have been selling the data submission drugs according to the regulations set by the government. As a result, the logic that the excessive number of generic items have made it difficult for the government to respond or manage GMP violations or detection of impurities such as NDMA and that a regulation tailored for large pharmaceutical companies that does not reflect the reality of small and medium-sized companies will be clashing at the Legislation and Judiciary Committee’s review. According to the National Assembly Act, the Legislation and Judiciary Committee only owns the right to review a bill(right to review the system and order self-revision) that has been already reviewed and passed by the relevant standing committee, however, in reality, there exists cases where the bill passed by the relevant standing committee remains halted at this stage without being processed. For example, the bill to revoke the license of a doctor who was sentenced to imprisonment or higher has been on hold for more than two months after the Health and Welfare Committee’s vote due to disputes between the ruling and opposition parties during the judiciary committee's review. In other words, whether the large and small- and mid-size companies will be able to reach a consensus on the bill may act as one variable for passing the bill. The Legislation and Judiciary Committee plans to review the 1+3 bill passed by the Health and Welfare Committee in July. If the bill passes this review, the final legislative process will likely be completed at the plenary session that will be held in the same month. An official who is in charge of development at a top domestic pharmaceutical company said, “The small- and mid-sized companies are arguing that the law should allow 4 or more pharmaceutical companies to jointly develop and produce the data submission drugs as its clinical trial costs 5 to 15 billion won. However, the fact is a clinical trial that costs 10 billion won is uncommon. If the companies are really opposing due to the burden of cost, they may receive recognition as a 1+3 bill exception according to the exception clause specified in the bill. The companies need to understand how serious the excessive generic and data submission drugs issue is and work to make remedies to their system.” Another official from a small- and mid-sized pharmaceutical company said, “Regulation on generics is necessary, but I oppose to the regulations being set on data submission drugs. The small- and mid-sized companies with insufficient power to develop new drugs yet need to have some possibility of joint development to be able to maintain management and make a cash cow with incrementally modified drugs. The bill is just another order for company restructuring. The bill will directly have an impact on reducing the number of pharmaceutical companies rather than the number of generic companies. This would inevitably lead to job cuts in the companies, so I cannot agree with a bill that disregards this reality.”
- Policy
- Gov will proactively improve system for severe psoriasis
- by Kim, Jung-Ju Jun 21, 2021 05:50am
- With a year left before the re-registration of special exemption of insurance calculation for severe psoriasis, the government, payer, and patient group gathered to discuss improvement. The issue discussed was that despite reimbursement approved for severe psoriasis drugs, patients are not being properly covered as the eligibility standards for the special calculation system are too strict, which increases the actual cost borne by patients. The patient group appealed to the government and payers that the standards for severe psoriasis should be set at the same level as other severe incurable diseases rather than solely relying on expert opinions, and the authorities said that they will take a proactive stance in resolving the issue. The Korea Alliance of Patients' Organization and the Korea Psoriasis Association had raised the issue at its 'Patient Shouting Cafe' event held on the 18th. Immediately after the event, the Ministry of Health and Welfare and the National Health Insurance Service held the roundtable and expressed their positions on improving the special exemption of insurance calculation for severe psoriasis. A patient group has requested improvement of the registration criteria for the exempted calculation of health insurance for severe psoriasis that will be up for re-registration in one year to the government and payers. Pic. from the hosted by the Korea Alliance of Patients Organization The point raised by KAPO was clear and simple. Patients with severe psoriasis have to bear a very high cost of treatment that can only be relieved through the special exemption of insurance calculation. However the entry criteria for the special calculation, the new and re-registration standards, are too strict, and only 17,500 out of the 22,000 severe psoriasis patients are unable to receive any benefit from the system. In other words, for most patients, the benefit is just a 'pie in the sky.' As the re-registration of special exemption of insurance calculation for severe psoriasis will start next year, unless the system is improved now through discussions, the issue is doomed to reoccur. However, the biggest barrier to the improvement is that the experts' opposition to making changes in the registration criteria. In fact, at the National Assembly's Health and Welfare Committee plenary session on the 16th, Yong-ik Kim, president of NHIS had answered Rep. Chun-sook Jeong's inquiry that as the payer, the organization had to follow the experts' judgment as its experts opposed to the change of new registration standards for special exemption of insurance calculation for severe psoriasis. On this, the patient group emphasized that when setting registration standards for special exemption of insurance calculation, policy judgments should be made based on equity with other disease and social consensus rather than simply by reimbursement standards. They argued that the special exemption system is different from medical insurance benefit standards that require consideration of cost-effectiveness and efficacy and that the government is hiding behind experts' judgment. High-priced biologics for severe psoriasis are already being reimbursed. However, contrary to other severe diseases like Crohn's disease, ankylosing spondylitis, and severe atopic dermatitis that apply special calculations immediately for high-priced biologics, the special calculation does not apply to severe psoriasis despite the reimbursement approved for its treatment. The high out-of-pocket payment borne by the patients greatly affects the livelihood and life of the patients. On this, the Korea Psoriasis Association, a direct stakeholder to the system, suggested that the NHIS transparently discuss this issue at the Special Exemption of Calculation Committee meeting, a discussion body for various stakeholders rather than blindly follow the judgment (medical judgment) made by the expert advisory committee. MOHW's Insurance Benefits Division and the NHIS responded that they will address the issue of new registration standards of severe psoriasis in a proactive manner and identify and find solutions for the issue that was discussed at the meeting.
- Policy
- Yuhan's Raboni-D has been licensed
- by Lee, Tak-Sun Jun 21, 2021 05:50am
- Yuhan, which had a high dependence on sales for imported drugs, has recently been speeding up with the commercialization of new drugs such as Lazertinib and IMD. In particular, Yuhan refrains from entrusting or entrusting developing products and is building market competitiveness with its own products. The MFDS approved "Raboni-D," Yuhan's combined osteoporosis IMD, on the 17th. It is a combination drug of Raloxifene HCl, an existing osteoporosis treatment drug, and Cholecalciferol Concentrated Powder, known as a vitamin D. There are already six products, including Hanmi Pharmaceutical's "Rabone D" and Alvogen Korea's "Ebistrar Plus," that combine the two ingredients. However it is evaluated that it has secured safety by lowering the amount of Raloxifene that was developed by Yuhan. Raboni-D is Raloxifene 45mg, while conventional products are Raloxifene 60mg. Raboni-D is used for the treatment and prevention of osteoporosis in postmenopausal women, and is given oral administration one tablet a day. Pregabalin SR, a pain reliever, is also a self-developed item by Yuhan Corporation. 15 items, including Pfizer's Lyrica CR, are licensed. Yuhan was also granted Yuhan Pregabalin SR in 2019. However, safety and effectiveness of Yuhan Pregabalin SR have not been confirmed in patients with new functional disabilities and those aged 65 or older compared to Lyrica CR. Yuhan is focusing on securing competitive edge in products that are as good as original by expanding clinical trials. In this clinical trial, pharmacokinetic characteristics and safety will be evaluated for patients with new disabilities and healthy people who have not been established safety and validity in the past when administering YHD119 single oral administration. Yuhan has been steadily decreasing its sales share of new drugs due to the expansion of its own development items. As of the end of the first quarter, sales of products stood at 56.6%, down 3.9% points from 60.4% in the same period last year. Analysts say that at this rate, the proportion of new drugs introduced in a few years will fall below 50%. The new drugs vary greatly with copyright agreements with developers, so there is a risk of maintaining sales. Leclaza was approved early this year, starting with the commercialization of the first IMD Duowell in 2014. Attention is focusing on whether Yuhan, which generated ₩1 trillion in sales for the first time in the pharmaceutical industry, will also gain fame as a new drug development company.
- Policy
- An exception to the 1+3 Bill for IMD
- by Lee, Jeong-Hwan Jun 18, 2021 05:54am
- A letter from a pharmaceutical representative to the National Assembly affected the process of the National Assembly's Health and Welfare Committee's handling of generics, drug for data-based re-evaluation "1+3 bill." It was reflected in the revised schedule when a representative of company A sent a petition to 24 members of the National Assembly's Health and Welfare Committee that IMD, which has decided to jointly develop more than four pharmaceutical companies, could have a negative impact on development and launch. The Pharmaceutical Affairs Law, approved by the National Assembly's Health and Welfare Committee on the 16th, contains the effective date, application case, and progress measures for medicines undergoing clinical trials. The most controversial part was the transitional provision of drugs already approved, or drugs already undergoing clinical trials, regardless of the Bill. If the bill goes into effect, pharmaceutical companies that exceed the four pharmaceutical companies regulated by the bill have pointed out that it is difficult to release drugs under joint development. In line with the implementation of the 1+3 bill, the welfare committee added an additional rule to apply the previous regulations to drugs under joint development after four pharmaceutical companies were approved by the MFDS. In this case, however, data proving the decision to jointly develop must be reported to the MFDS within one month from the enforcement date of the law. The amendment also reflected claims contained in a petition letter from the representative. In a letter, he explained that IMD, an arthritis drug that the company is jointly developing with several other pharmaceutical companies, is on the verge of being suspended with the 1+3 bill. He added that IMD, which requires billions of won, is conducting Phase 3 clinical trials by investing development costs jointly with multiple domestic companies because it is difficult for small and medium-sized pharmaceutical companies to invest R&D costs alone. During the development process, the 1+3 regulation bill passed the welfare committee on April 29, and he stressed that his company was in danger of having to stop developing IMD research and development because of the bill. "We can accept the general regulations to prevent the disturbance of generic drugs," he said in a letter. "The IMD's regulations can be difficult to develop without a certain period of delay," he said. "The IMD has already received development costs from multiple domestic companies and is in the process of Phase III clinical trials." The welfare committee partially accepted his intention of IMD and established an additional clause that submits and reports evidence that he is jointly developing it to the MFDS within a month of the enforcement of the law. An official of the welfare committee said, "We collected opinions to minimize confusion among pharmaceutical companies that have been preparing for IMD development through joint development contracts regardless of the bill." "We have resolved unreasonable parts and improved the completion of the bill with the provision of transitional measures for medicines undergoing clinical trials," he explained.
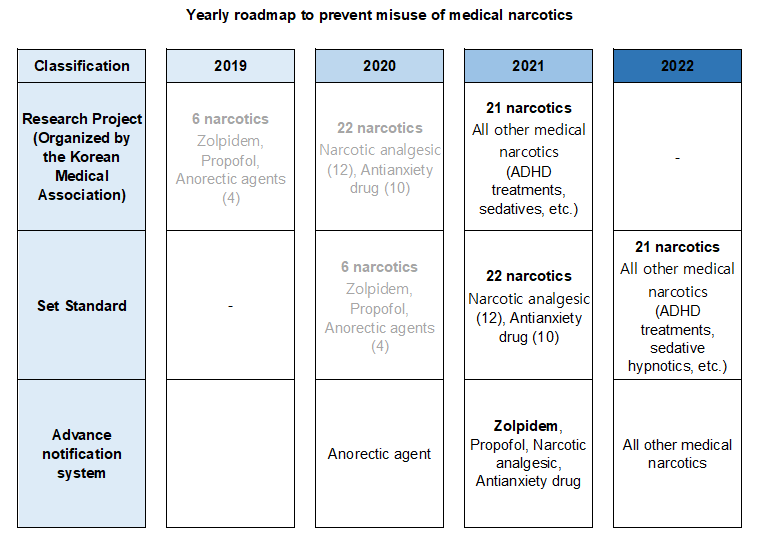
- Policy
- 559 doctors warned for wrongful prescription of Zolpidem
- by Lee, Tak-Sun Jun 18, 2021 05:53am
- After analyzing the prescription information on the Narcotics Information Management System (NIMS), the Ministry of Food and Drug Safety announced its decision to issue written ‘warnings’ to 559 doctors that have continuously prescribed or used Zolpidem beyond the safe use standards to prevent abuse and promote proper use of the medical narcotic, Zolpidem. This measure is the second step taken by regulators based on analysis results of the 2-month prescription and use history that was collected after notifying the 1,720 doctors who prescribed and used Zolpidem beyond the safe use standards on March 2nd, through the advance notification system as the first step. MFDS said that the doctors' compliance rate of medical narcotics safety guidelines had improved after implementing the advance notification system. The number of doctors who prescribed and used Zolpidem beyond the set safe use standards after receiving the first step warning in March decreased 68% from 1,720 to 559, and the total number of prescriptions also decreased 51% from 5,593 to 2,724. Also, the ministry added that it plans to conduct on-site inspections and take administrative actions (suspension of handling narcotics) to restrict prescriptions of doctors who do not correct their actions in prescribing medical narcotics beyond the scope set under the safe use standards even after the second written warning. An MFDS official said, “The advance notification system that has been implemented to anorectic agents in December last year will also be applied to analgesics and anti-anxiety drugs this year. By next year, we plan to expand the system to all narcotics, through which we aim to continue our efforts in preventing misuse and abuse whiling encourage safe use of medical narcotics in Korea.”
- Policy
- “The 1+3 bill” was passed by the Welfare Committee
- by Lee, Jeong-Hwan Jun 18, 2021 05:53am
- The 1+3 bill passed a plenary session of the National Assembly's Health and Welfare Committee on the morning of the 16th and will be reviewed by the legislation and judiciary committee. The resolution reflected the revision of the supplementary provision, which excludes the report from the MFDS within a month from the enforcement date of the law, a drug for data-based re-evaluation, which was approved before the enforcement of the law. The welfare committee has passed on a number of the pharmaceutical affairs law to the judiciary committee, including the bill, mandatory preparation and submission of expenditure reports on pharmaceutical CSO, designation of drug days as a legal anniversary, and mandatory safety vulnerable braille and voice codes. A total of 94 bills were handled by the welfare committee on the same day. The most interesting legislation is the 1+3 bill. The main point of the bill is to allow up to three consignees per trustee to conduct joint biological equivalence tests and clinical trials necessary for the development of drug for data-based re-evaluation with generic. Regardless of the revision of the bill, the welfare committee partially reflected the transitional measures for medicines that are already undergoing clinical trials. They say that drugs that have signed a joint development contract and received approval for a clinical trial plan from the head of the MFDS do not apply the 1+3 regulation. In this case, however, pharmaceutical companies that have been approved for clinical trials must submit data proving the joint development of drugs to the MFDS within one month from the enforcement date of the law. Like pharmaceutical companies, CSO, which is in charge of promoting drug sales, has also been designated as a drug supplier, imposing obligations to prepare and submit expenditure reports for doctors and pharmacists, and regulating those who illegally purchased Rx drugs. The welfare committee passed on amendments to the Infectious Diseases Act, the Medical Devices Act, the Infectious Disease Prevention and Control Act and the Health Insurance Act to the legislation and judiciary committee.The welfare committee introduced 114 new bills to the plenary session to continue its review at the legislation and judicial committee. They included bills that surveys and public announcements on the status of illegally licensed pharmacies, hospitals run illegally by the office manager and illegally licensed pharmacies( they will be excluded from health insurance care institutions), vaccine development status sharing, paid leave after vaccination, expanded national coverage for side effects, and prohibition of the establishment of a medical institution for child sex offenders.
- Policy
- Moderna vaccine has been approved for lot release
- by Lee, Tak-Sun Jun 18, 2021 05:53am
- The MFDS said it approved lot release of 55,000 doses of Moderna COVID-19 vaccine on the 15th. The national lot release is a system in which country evaluates the results of the verification test and the review of the manufacturer's data before the vaccine is distributed on the market and checks the quality once more. The MFDS measured efficacy based on genetic materials and the amount of lipid nanoparticles surrounding them through invitro translation of the first import of Moderna COVID-19 vaccine. The purity test and endotoxin test confirmed that the vaccine was safe because it was not contaminated. The MFDS explained that quality test data issued by the plant quality assurance director were reviewed to ensure consistency in quality. Moderna vaccine is licensed and distributed by GC Pharma. GC Pharma also applied for the national lot release. Starting this week, Moderna vaccine will be inoculated to health care workers under the age of 30, medical students, and nursing students from general hospitals. When Moderna vaccine is used on site, the total number of vaccines inoculated in Korea will be increased to four, including AstraZeneca, Pfizer and Janssen.
- Policy
- AZ vaccine's side effects are reviewed
- by Lee, Tak-Sun Jun 17, 2021 08:02pm
- The MFDS has begun a review on the addition of side effects of capillary leakage syndrome of AstraZeneca's COVID-19 vaccine recommended by the European Medicines Agency (EMA). The MFDS announced on the 14th that it will take necessary measures such as distributing Dear Healthcare Professional Letter, sharing information recommended by EMA, and adding related precautions to Insert paper. Earlier, the Pharmacovigilance Risk Assessment Committee (PRAC) of the EMA recommended that people with a history of capillary leakage syndrome should not be vaccinated against AstraZeneca's COVID-19 vaccine. The PRAC reviewed 6 cases of capillary leakage syndrome in people who received AstraZeneca's COVID-19 vaccine, with 3 having a history of capillary leakage syndrome, and one of them fatal. Capillary leakage syndrome is a very rare and serious condition that causes fluid leakage from the capillaries, resulting in edema of the arms and legs, low blood pressure, blood concentration and hypoalbuminemia. No patients have yet been reported in Korea. The MFDS shared relevant information with medical experts, saying that people who have experienced capillary leakage syndrome should not be vaccinated with the AstraZeneca's COVID-19 vaccine. It also said it will take necessary measures such as adding related precautions to the insert paper by combining the status of abnormal cases at home and abroad and safety information.
- Policy
- PBAC provides cost-effectiveness data to improve integrity
- by Lee, Hye-Kyung Jun 17, 2021 06:04am
- The Health Insurance Review and Assessment Service's Pharmaceutical Management Department was selected as 'best project' among 'self-promoted projects to improve integrity’ for its efforts in improving standard forms and expanding information disclosure systems. The department worked to improve integrity by clarifying and making transparent the application process for the decision-making of pharmaceutical benefits. Specifically, the department expanded the scope of information disclosed from the Pharmaceutical Benefit Appraisal Committee (PBAC) meeting to the cost-effectiveness data. In the past, the data shared after the meeting was focused around clinical efficacy data. The system was improved so that the PBAC evaluation results, which were provided over the phone upon the company’s request, are now provided for all pharmaceuticals that were deliberated, immediately after the meeting without requests from relevant pharmaceutical companies. In addition, the department has been commended for improving work efficiency by clarifying and standardizing the data required for submission when applying for the pharmaceutical benefit. Also, the department developed a status tracking service after applying for the extension of the benefit standard, which subdivides the review process into 7 stages - confirmation of receipt, supplementary data request, academic opinion request, practical review, committee deliberation, report to the Ministry of Health and Welfare, and process completion - to be checked by the applicant. In addition to the Pharmaceutical Management Department, the integrity promotion projects proposed by the Safety Management Department, Customer Services & Public Relations Department, Claims Review Department, and Medical Fee Schedule Department were also selected as Best Projects. HIRA has been making efforts to improve the integrity of its organization through various efforts including raising integrity awareness of its internal employees and enhancing communication with partner firms, improving employee satisfaction by achieving worker-customer balance, holding a mutual respect day, establishing and operating an anti-corruption relief program, promoting a Clean&Clear Cost (3C) system, preparing criteria for handling out-of-town business trips, etc. At the press briefing held on the 15th with the Korea Special Press Association, Shin Cho, standing auditor of HIRA said, “Analysis of the integrity score last year showed that the institution recorded high scores in illegal solicitation, bribery, and private use of budget categories, but scored relatively low in the organizational culture category. To increase the integrity of our institution, we will work to establish a healthy organizational culture and strengthen the virtuous circle for anti-corruption and integrity."
- Policy
- Ruling/opposition parties fight over supply & COVID vaccine
- by Lee, Jeong-Hwan Jun 15, 2021 05:53am
- Political circles of the ruling and opposition parties have launched a special committee on the COVID-19 vaccine and TF respectively, and are checking each other over the status of vaccine supply and vaccination performance. The ruling party has been announcing the results of COVID-19 vaccine since the Korea-U.S. summit, while the main opposition party has criticized the government for pointing out the issue of vaccine supply and demand and disruption to its goal of mass immunization in November. The ruling and opposition parties are busy with their respective activities on the COVID-19 vaccine special committee and TF, which were launched on the 14th. Currently, the Democratic Party of Korea has launched and operated the COVID-19 vaccine and treatment special committee, and the People Power Party has launched and operated the COVID-19 vaccine TF. The Democratic Party of Korea appointed Jeon Hye-sook(pharmacist), as its chairman, and Kim Sung-joo, an executive secretary of the Health and Welfare Committee, as its members, and Seo Young-seok(pharmacist). The People Power Party TF, chaired by the Health and Welfare Committee's executive secretary(Kiyoon Kang), consists of a total of 10 members, including Seo Jung-sook(pharmacist), Kim Mi-ae, Lee Jong-sung, Cho Myoung-hee, lawyer Park Joo-hyun, director Lee Sang-ho of the Korea Medical Association, and Choi Jae-wook. With the launch of the special committee, the ruling and opposition parties have begun to check the supply and demand of COVID-19 vaccine, which is the nation's biggest pending issue, and the current status and strategy of the national vaccination. Specifically, the Democratic Party of Korea held its third meeting on the 14th of this month following its first meeting on the 24th of last month to follow-up measures on COVID-19 vaccines and treatments and check major status of vaccines. In particular, the Democratic Party of Korea is determined to invite domestic pharmaceutical and bio companies and government ministries such as SK Bioscience, Huons, and Samsung Biologics to prepare effective domestic industrial support measures at the second meeting. At its first meeting on the 8th, the People Power Party TF decided to determine the exact cause of the current administration's failure to supply and demand COVID vaccines and aggressively reveal the reality that it is difficult to form a mass immunity (with a vaccination rate of more than 70%) in November. In fact, the People Power Party TF took issue with the fact that the original government budget did not reflect the purchase cost of the vaccine at all, and that the inoculation rate to the population was only 9.1% as of the 28th of last month. Furthermore, TF plans to urge the government to improve its quarantine system along with support for domestic pharmaceutical and bio industries such as securing vaccines in Korea, supporting production of consignment and self-development, and improving quarantine systems. The ruling and opposition parties are not just checking each other over COVID-19 vaccine. The ruling and opposition parties held a public hearing on the 14th to promote the formation of a special parliamentary committee to support the global vaccine hub and decided to work together to review the necessity and validity of the joint committee. It is aimed at serving as a medium for promoting cooperation between the government and businesses as South Korea's potential to leap forward as a global vaccine hub has been confirmed in the wake of the recent Korea-U.S. summit. Democratic Party of Korea Corona Vaccine Special Committee (top) & People Power Party COVID Vaccine TF The special committee aims to enhance domestic vaccine development capabilities, strengthen diplomatic capabilities to promote international vaccine cooperation, and enhance vaccine technology capabilities by establishing a global vaccine partnership between South Korea-U.S. The public hearing was co-hosted by Democratic Party of Korea Yang Ki-dae and People Power Party Choi Hyung-doo, and presented by Hanmi Pharm CEO Lim Jong-yoon and ST Pharm CEO Kim Kyung-jin. It is true that the joint public hearing has created a possibility of a joint special committee between the ruling and opposition parties for COVID-19 vaccine, but whether it will be realized depends on whether the leaders of the party will form a consensus. "Since the ruling and opposition parties have recently elected a new party leader and floor leader, it is not easy to form a consensus on the supply, vaccination and national quarantine of COVID-19 vaccines," a National Assembly official said. "The structure of forming a special committee and a TF to check will continue for the time being.However, the move to collect opinions from the pharmaceutical and bio industries through public hearings will be made together with the ruling and opposition parties and reflected in the industry promotion and vaccine hub policies," he said.