- LOGIN
- MemberShip
- 2025-12-24 12:42:40
- Policy
- Suspension of Execution for Xarelto price cut continues
- by Kim, Jung-Ju Jul 05, 2021 05:54am
- Despite the two price cuts that were previously notified from the ex officio adjustment and expiry of the premium pricing application period, the price of the 4 strengths of Bayer Korea’s Xarelto tab. products will continue to be maintained as is for the time being. The company, which filed a suit against the government regarding the price cut, also requested for a suspension of execution of the price cut during the trial period, which the court accepted. On the 2nd, the 11th division of the Seoul High Court ordered the suspension of execution of Xarelto’s price cut until the 30th day from the date of the judgment made for the case the company filed against the Ministry of Health and Welfare to cancel the price cut disposition for Xarelto (No. 2021 Guhap 765811). The suit was filed after the Ministry of Health and Welfare included the 4 strengths of the Xarelto tab. products as items that will undergo price cuts as an ex officio adjustment and be up for termination of premium pricing in May. The government has been making ex officio adjustments to the original drug as well as the generic with the same route of administration, ingredient, and formulation when generics are listed. When the first generic is listed, the price of the original is adjusted to 53.55% of the original price but given a premium of 70% of the original price for 1 year. Previously, the government had issued a notice confirming the price cut of Bayer Korea’s Xarelto, effective as of June 1st. Prices of Xarelto tab. (rivaroxaban) 10mg, 20mg, 2.5mg were to be cut by 30% each, and then further lowered by 23.5%-23.6% one year later, from May 1st, 2022. With the court's decision to suspend execution, the drug price of the Xarelto tab. will remain the same for the time being, and no change will be made in medical institutions as well. However, if the government wins the case, the price cuts will again be in effect, which will once more be restored to the original price if the company appeals the case. Therefore, the possibility of price fluctuation remains. According to the government, the MOHW has never lost drug price-related lawsuits like Xareolto's. Due to this, price fluctuation of drugs during the back-and-forths has been trending in similar suits. The MOHW announced that the price maintenance will be applied as of the 2nd and that the ministry will share additional information if any further changes are made.
- Policy
- The price of 7 Seretide products will be reduced
- by Kim, Jung-Ju Jul 05, 2021 05:53am
- The government won the suit. But there is a possibility of change depending on the company's decision. Insurance prices for 7 items of asthma treatment Seretide by GSK will be lowered from the 2nd of next month. The government won the lawsuit against the company to cancel the reduction of the price. If the company disagrees with the ruling, there may be another change in drug prices. The sixth part of the Seoul Administrative Court sided with the MOHW in a lawsuit filed by the company. Therefore, suspension of execution, which had maintained the previous price without lowering the drug price during the lawsuit period, was lifted. The lawsuit was taken by the Ministry of Health and Welfare on June 1, 2019, in line with the end of the additional period of these products, on May 28, 2019. The company filed a suspension with the court and applied for a suspension of execution to keep the drug price intact during the lawsuit period, but it was lifted with the ruling. These drugs include Seretide 125 Evohaler(120 doses), Seretide 100 Diskus(60 doses), Seretide 250 Diskus(60 doses), Seretide Evohaler 250(120 doses), Seretide 250 Diskus(28 doses), Seretide 500 Diskus, Seretide 50 Evohaler(120 doses) . The MOHW said it would apply the reduction as of July 2 in accordance with suspension of execution. However, there is a possibility of change if the company disagrees. In response, the MOHW has decided to provide additional guidance if there is any change in the future.
- Policy
- The reasons why Hemlibra's benefit was not recognized?
- by Lee, Hye-Kyung Jul 03, 2021 05:56am
- The HIRA's Central Review and Coordination Committee revealed specific reasons for not recognising benefit of JW Pharma's Hemlibra (Emicizumab). The HIRA released a June review of the Review and Assessment Committee on its portal on Thursday. There are five cases of deliberation, including Hemlibra, Soliris, ventricular assistive therapy, Spinraza, and hematopoietic stem cell transplantation. In the case of Hemlibra, the HIRA unusually disclosed the results on the 8th due to controversy over the standards. Based on the data submitted by the medical institution, the central group said that cases A, B, and C lacked objective data to prove that it was difficult to secure venous blood vessels and to insert and maintain central vein tubes. ▲For pediatric administration, patients with failed Immune Tolerance Induction,▲ If there is a doctor's statement that it meets the requirements subject to Immune Tolerance Induction but is not possible to carry out it,▲ If antibodies are regenerated after the success of Immune Tolerance Induction, the patient must pay the full copayment of Hemlibra. According to the case of disapproval, which was specifically disclosed to the medical institution's business portal, A case was diagnosed with hereditary 8th factor deficiency syndrome in June 2019 and antibodies were found in December 2019 and started administering Hemlibra through clinical trials in March 2020. In February this year, the patient met the criteria for Immune Tolerance Induction benefits, but claimed Hemlibra because it was very difficult to secure venous blood vessels and difficult to insert and maintain central venous tubes as antibodies to factor VIII. However, the HIRA did not acknowledge the benefit, saying that "submitted medical records show insufficient objective data to determine that Hemlibra administration in February 2021 is difficult to secure venous blood vessels and to insert and maintain central venous ducts." Case B is a male (22 months), diagnosed with factor VIII disease in August 2019 and antibody was found in June 2020. The patient also applied for Hemlibra benefits because of the very difficulty in securing venous blood vessels, difficulty in inserting and maintaining central venous tubes due to antibodies to factor VIII, and psychological problems such as fear of intravenous injections, but was not recognized for the same reason as case A. There was also a lack of medical explanation for "stress from a vein injection". Case C was a 4-year-old boy, diagnosed with factor VIII disease in June 2016 and discovered antibodies in July 2016, but had a history of treatment in intensive care due to subgaleal hemorrhage and epidural hemorrhage at birth. The HIRA judged that "the past history of severe bleeding is not the case when immunotherapy cannot be attempted." Case D is a 3-year-old (40 months)-old male diagnosed with factor VIII disease in September 2017 and antibody was discovered in November 2017. In November 2018, chemoport was approved, but three days later, intestinal bleeding failed, and Hemlibra was charged for failing to be administered due to severe intestinal bleeding in February 2021. However, the HIRA ruled that "Immune Tolerance Induction could not be performed due to intestinal bleeding in November 2018, but medical records confirm the application for IImmune Tolerance Inductionagain in January 2019." Meanwhile, the MOHW announced that it would review the benefit standard in consultation with the HIRA as problems with the Hemlibra standard were pointed out at a plenary session of the National Assembly on the 16th.
- Policy
- Rationalizing ICER threshold requires various considerations
- by Kim, Jung-Ju Jul 01, 2021 05:56am
- Yoon-Seok Yang, Director of MOHW’s Division of Pharmaceutical Benefits Insurance authorities expect the evaluation results of the listed drugs that underwent quality reassessment on the reasonableness of their medical care benefit (re-evaluation of medical benefits of listed drugs) will be applied as early as October this year. This means that the results will pass the Health Insurance Policy Deliberative Committee’s review and be notified in October. Also, regarding the issue of Keytruda’s reimbursement expansion that has been making little progress, the government has pressed for a clear financial sharing scheme with measures such as initial refunds. On lowering the drug price in the detailed evaluation criteria of drugs up for drug pricing negotiations to 80% of the lowest A7 adjusted price, the government plans to continue to hold an ear out to the industry and accept acceptable opinions. This was what Yoon-Seok Yang, Director of Ministry of Health and Welfare’s Division of Pharmaceutical Benefits said in response to the Korea Special Press Association’s questions on pending issues. Director Yang candidly shared the authorities’ positions and concerns over various pharmaceutical policies in his division ranging from reimbursement entry, expansion, improving reimbursement accessibility of ultra-expensive drugs, to ICER thresholds and other various reevaluation issues related to new drug listings. The following is a QA of an interview with Director Yang New drug listings – regarding ICER and PE exemption, RSA, and detailed criteria for drug pricing negations ▶The industry has been requesting rationalization of the ICER threshold in line with the $30,000 GDP per capita era. The ICER threshold is already being flexibly applied on severe and rare diseases, but the industry seems to believe that the threshold should be set at least 3 GDP. “The issue has come up in the NA audit and has also been requested by the industry. As far as I know, HIRA is currently reviewing the issue. Accessibility to new drugs is directly related to drug price, and from the government’s perspective, directly related to NHI finances, so this issue needs to be approached with caution. The fact that you use the term ‘rationalize’ seems to imply that the current ICER threshold is unrealistic. However, there are others who believe that the current threshold is reasonable. We need to gather opinions from various sides before implementing any measures because the drug price also affects reimbursement expenditures. Many drugs have been receiving reimbursement benefits through the PE exemption system without ICER evaluations, so when the government collectively considers its finances, these drugs also need to be taken into account in this discussion. As I said, we need to first gather various opinions. We also need to bear in mind that not many countries use ICER thresholds directly connected to GDP.” ▶There was also talks in the industry that HIRA would lower the PE exemption criteria to 80% of the lowest A7 adjusted price while revising some phrases in HIRA’s evaluation criteria for negotiated drugs. In detail, the criteria would, in principle, set the rate at 80%, and some at 100% according to each drug’s characteristic. Multinational pharmaceutical companies have been greatly opposed to this change, and the question of whether the system and mechanism would become a ‘dead letter’ is also being raised. Is there any room for adjustment on this matter? “We will listen, review, and accept reasonable arguments to the extent possible. The government and HIRA are both aware that the ‘visible’ price listed for most RSA drugs is not the ‘actual price.’ Our focus is on whether the price should be accepted as is, or be adjusted to reflect the real price. The industry will not be able to deny that this is a serious issue. But we will keep an ear out for suggestions from the industry.” ▶Considering that over half of the high-price drugs receive reimbursement through the PE exemption scheme, did the ministry lower the PE exemption criteria to 80% of the lowest adjusted price because the PE exemption scheme is being used more actively than expected?. “Drugs need to be evaluated and given a price according to their value. We know that it is very difficult for rare disease drugs to prepare data for such evaluations, and that’s why we have the existing tools that strengthen post-management measures. HIRA wishes to find measures to strengthen the criteria through discusstions with the industry, but the industry is pushing their opinion. They need to discuss this together. The PE exemption scheme increases payment on HIRA’s part, so HIRA believes in the need to strengthen its management.” Tecentriq and Keytruda ▶Tecentriq applied for reimbursement separately and is up for deliberation by the Cancer Drug Review Committee. I heard that the two drugs will be now discussed together, and the key to Keytruda’s issue is that it lacks a cost-sharing scheme. As the government’s signal would play an important role in the decision, the question that remains is - is the initial refund is crucial?. "Under the premise that the clinical efficacy has been demonstrated, in general, the payer and the pharmaceutical company’s actions are what makes or breaks reimbursement expansions. In other words, the discussion and negotiations on how to share the financial burden are what take up the longest time. A slow progress like Keytruda's is a first for us in our history as well. I cannot disclose specific numbers, but even after CDRC's recommendation to take up the financial sharing request, pre-negotiations fell through. The government's request for a financial sharing scheme similar to those made for other drugs also broke down. The company just increased the scope of its indications and reapplied for reimbursement. This financial-sharing issue is still being discussed, and I hope the company would take a more forward-looking stance regarding this matter. The reason why the authorities emphasize the initial sharing plan is because it allows for a clearer sharing of cost during the initial period where the increase in reimbursement expenses from the expansion is unclear. Initial sharing of cost eliminates financial uncertainty on the government’s part. I know that the company is also seriously considering this matter, and hope that an agreement can be reached in the near future. ▶When will the agenda be introduced?. “The agenda will be introduced sequentially according to HIRA’s order of placement. I believe it will be introduced around July or August.” Premium pricing reevaluation ▶The issue at hand is the premium pricing of single-brand drugs. As brought up during the last pharmaceutical-HIRA roundtable, even when after applying for adjustments, the possibility of accepting the adjusted price is low and it is realistically difficult to reach an agreement, which eventually leads to discussions of taking legal actions. Wouldn't it be resolved by adjusting the timing, by allowing for adjustment applications to be submitted during the 60-day negotiation period set for the stable supply obligation so that they can reach an agreement earlier? “We are reviewing various options. We are trying to adjust the price according to our system, but if the companies say the price is unsuitable for supplying the drug, I think it is right to adjust it to a reasonable price. Some parts of the current standard seem to be strict, and there are other parts where HIRA is not properly carrying out its assessment, and we are determined to address and improve these parts. However, as addressing these issues will increase the drug price, it is natural for the government to be precautious of implementing such price-increasing mechanisms because it will increase the burden on NHI finances, so we are seriously reviewing options with HIRA. Receiving applications during the negotiation period is not being reviewed under current circumstances. However the re-evaluation of premium pricing is being implemented due to a change in the law and system, and I hope the industry would accept this. After the grounds are fully justified, we will seek measures to adjust the price of the drugs.”
- Policy
- Targets for PVA in third quarter include Darzalex & Alunbrig
- by Lee, Hye-Kyung Jul 01, 2021 05:55am
- Janssen Korea's (Daratumumab) and Takeda Korea’s Alunbrig (Brigatinib) were included in the Price-Volume Agreement (PVA). Otsuka Pharmaceutical Korea's "Iclusig" and AstraZeneca Korea's "Impinji" (Dubalumab) were also subject to negotiations on drug prices due to increased usage. The NHIS recently introduced "PVA (Type Ka/Na) Monitoring Drugs for 3rd Quarter of 2021" on its website. Targets for monitoring in third quarter of this year are 173 items from 90 pharmaceutical groups. PVA is a method shared by the NHIS and pharmaceutical companies to share the risk of health insurance finances, and the drug price will be reduced through negotiations with the NHIS. Type Ka of PVA corresponds to cases in which claims for the same product group with expected claims agreed with the NHIS have increased by more than 30% from expected claims. Type Na is subject to the same product group, which has been negotiated for type Ka or not negotiated for type Ka, where the previous type has increased by more than 60% or more than 10% or ₩5 billion every year from the end of the analysis period. Drugs with an annual claim of less than ₩1.5 billion, drugs with lower upper limit than the arithmetic average of the same ingredients, low-cost drugs, and drug shortage prevention programs are excluded from PVA. The pharmaceutical groups included Samoh's "Vimizim (Elosulfase Alpha)," Sanofi-Aventis Korea's "Mozobil (Plerixafor)," BL&H's Trisenox(Arsenic Trioxide) and SK Chemical's Migard(Frovatriptan Succinate Monohydrate). The pharmaceutical groups included Samo Pharmaceutical's "Vimizim (Elosulfase Alpha)," Sanofi-Aventis Korea's "Mozobil (Plerixapor)," BL&H's "Arsenic Trioxide" and SK Chemical's "Migard" (Frovat Monochlithinccate).
- Policy
- 2 indications deleted for choline alfoscerate, changes label
- by Lee, Tak-Sun Jun 30, 2021 05:56am
- Authorities have ordered companies to delete the No.2 and No.3 indication of the brain function enhancer choline alfoscerate, for which the clinical re-evaluations have started recently. Accordingly, the indication must be removed from the label from the 28th of next month. Also, the Ministry of Food and Drug Safety requested doctors and pharmacists to review the need to prescribe and dispense substitute drugs for patients that use choline alfoscerate during the labeling change period. On the 28th, the MFDS ordered a label change for 144 choline alfoscerate products. After a review of the clinical trial protocols for the reevaluation of these products, the ministry determined that that trial will be unable to assess the efficacy of choline alfoscerate’s second and third indications and decided to remove the two indications. As a result, only the first and main indication, for ‘Secondary symptom caused by cerebrovascular defects or degenerative brain-organic psychiatric syndrome by cerebrovascular deficiency: reduced memory, derangement, disorientation by reduced willingness and spontaneity, reduced willingness and spontaneity, reduced concentration’ will be left approved for choline alfoscerate. The second indication, for ‘Emotional and behavioral change: emotional insecurity, sensitive to stimulation, indifferent to surrounding,’ and the third indication for’ Senile pseudodepression’ will be deleted. However, whether the first indicating will remain intact depends on the results of the domestic clinical reevaluation that will be conducted. The MFDS had ordered companies to submit clinical trial results for Alzheimer's patients by December 9th, 2025, and the same for patients with mild cognitive disorder by March 9th, 2025. 57 companies including Daewoong Pharmaceutical and Chong Kun Dang that will lead the clinical trial will be participating in the clinical reevaluation. With the order to change labels issued, choline alfoscerate products that will be produced a month from the indication change, from July 28th, must reflect the new efficacy and effect on the product box and label. Administrative sanctions will be imposed on the companies that do not comply with the order by the said date. In 2019, the prescription sales amount of choline alfoscerate for the deleted two indications (No.2, No.3) amounted to 39.3 billion won and accounted for 11.1% of total prescription sales for the drug. Therefore, pharmaceutical companies are not expected to suffer huge performance losses from the change of indications. The companies are more intently focused on negotiations with the government on the amount they may have to give back in case the reevaluations for the first indication fall through. The National Insurance Health Service had recently proposed a retrieval rate of 30% of the insurance claims amount to make progress in the fruitless negotiations.
- Policy
- Will Rosuvastatin OD be released?
- by Lee, Tak-Sun Jun 30, 2021 05:55am
- Crestor (Rosuvastatin)Attention is focusing on whether a treatment for hyperlipidemia, Rosuvastatin OD (Orally Disintegrated Tablet) will be released. Rosuvastatin OD was approved in Japan in 2016, but has not yet been released in Korea. According to industries on the 25th, BCWORLD Pharm recently applied for approval of Rosuvastatin OD to the MFDS. AstraZeneca's Crestor is the original for Rosuvastatin. Generics have been released in Korea since 2009, and 941 cases are currently approved. However, there is no formulation of ODT that is melted with the tongue without water. In Japan, AstraZeneca and Shionogi were approved for Crestor OD in 2016. It is said to have been developed by Shionogi. ODT is useful for patients who have difficulty swallowing tablets or who need to refrain from drinking water. However, since most patients are familiar with regular tablets, there will not be much demand for ODT formulation. Last year, Crestor recorded ₩85.5 billion in outpatient prescriptions in the country. Therefore, if ODT is released for the first time, it is enough to attract attention regardless of market size. However, it is too early to discuss the success of commercialization. When a new drug is applied for permission, the MFDS decides whether to approve the sale after safety and the review.
- Policy
- Janssen Vaccine for 10,800 people has been approved
- by Lee, Tak-Sun Jun 29, 2021 05:46am
- The MFDS said it approved the nation lot release on the 25th (Friday) for 108,800 people of "Covid-19 Vaccine Janssen" applied by Janssen Korea. Nation lot release is a system in which the state checks the quality of the vaccine once again before it is distributed on the market by comprehensively evaluating the MFDS' test results of the vaccine. The MFDS explained that it has been thoroughly preparing for rapid national lot release of "Covid-19 Vaccine Janssen" by verifying its own test method since early this year and introducing equipment needed for testing such as enzyme analyzers. Test methods established by the MFDS include implantable gene expression, viral gene verification, viral protein verification, vector content, and purity. The effectiveness, safety, and quality were confirmed through the test and manufacturing and test data review of Covid-19 Vacine Janssen Inj and the lot release was decided because it met the national shipping approval criteria. This product was tested for the effectiveness of vaccines such as ▲ antigen protein expression, viral gene confirmation indicating effectiveness, and viral gene and transporter (vector) content by conducting a ▲ antigen test, verification test, etc. ▲It was confirmed that the product was not contaminated by conducting aseptic test, endotoxin test, and purity test. ▲ The MFDS explained that quality consistency was confirmed by conducting quality, pH, and practicality tests and reviewing quality test data issued by the manufacturer's quality assurance manager. Covid-19 Vaccine Janssen Inj is a virus vector vaccine manufactured by incorporating COVID-19 surface antigen gene into the adenovirus mold, the same platform as the country's first licensed AstraZeneca Korea Covid-19 Vaccine Inj. The MFDS stressed that it will do its best to thoroughly verify COVID, which will be introduced in Korea, by making the most of the related infrastructure necessary for the national lot release. Janssen vaccine, which was approved for lot release this time, is not a vaccine donated by the U.S. government, but an individual contract with Janssen.
- Policy
- Leclaza was listed at ₩68,964 per tablet
- by Kim, Jung-Ju Jun 29, 2021 05:46am
- Leclaza 80mg (Lazertinib) made with domestic technology passed the final step to insurance benefits. Leclaza finally chose this track, considering its similar clinical utility to the alternative drug Tagrisso, but cheaper with the Risk Sharing Agreement (RSA) Refund and Expenditure Cap. The date will be started on July 1, which means that the company succeeded six months after the approval in January. The MOHW announced on the 25th that the revised "Pharmaceuticla benefit list and upper price limit table" was proposed as a sub-issue of the 15th Health Insurance Policy Committee. Leclaza is a third-generation epithelial cell growth factor receptor (EGFR) tyrosynkina inhibitor (TKI) and received "conditional approval" from the MFDS on January 18 this year as a treatment for local progressive or metastatic non-small cell lung cancer. At that time, the company applied for insurance registration at the HIRA on December 30 last year, just before being licensed using the drug approval-patent linkage system. On 24 February, the HIRA held the Cancer Drugs Benefit Appraisal Committee to discuss the drug, and was judged on 8 April after deliberation by the Pharmaceutical Benefits Advisory Committee. The Pharmaceutical Benefits Advisory Committee determined that the clinical usefulness was similar to that of Tagrisso, an alternative drug, and that it was therapeutically equivalent to Tagrisso, which is currently being applied to RSA. Leclaza also concluded that following the RSA track would be cost effective at a lower cost than Tagrisso. Korean Cancer Study Group, Korean Society of Medical Oncology, and Korean Association for Lung Cancer suggested these opinions that similar effects have been shown compared to Tagrisso, and it is an additional treatment alternative that demonstrates safety results such as decreased cardiac toxicity and is more effective than treatment. It chose refund and expendature cap among RSA. The RSA Refund type is that a pharmaceutical company reimburses the NHIS for the full amount of pre-determined drug claims, and the Expenditure Cap reimburses the NHIS for a certain percentage of the excess if the actual claims exceed the pre-set annual expected cap. Since then, the MOHW has ordered negotiations on drug prices between the NHIS and the company and agreed on the estimated amount of claims for Refund and Expenditure Cap from April 23 to the 8th of this month. It has agreed that it will make $18.5 million (₩14.1 billion). The NHIS predicts no additional financial needs because there is an alternative drug, Tagrisso. Leclaza was insured from authorization in just half a year. The price is ₩68,964 per tablet, and the benefit will begin in earnest on the 1st of next month.
- Policy
- Following Gilead's hepatitis C tx Epclusa, Vosevi was filed
- by Lee, Tak-Sun Jun 28, 2021 05:50am
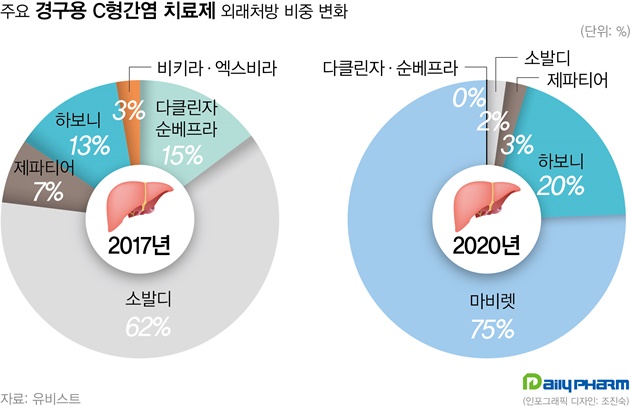
- This year, hepatitis C drugs Epclusa and Vosevi have been filed by the MFDS in Korea. These items are expected to replace Sovaldi. According to the industry on the 27th, Gilead's Vosevi (Sofosbuvir/Velpatasvir/Voxilaprevir) has recently been filed and will begin screening in earnest. Vosevi is expected to be a Pangenotypic DAA preparation that can be used in patients who fail to treat existing Direct-Acting Antiviral (DAA) drugs. AbbVie's Mavyret, a phangenotypic inhibitor, is now the market leading item. And, Mavyret is applicable to both chronic hepatitis C virus genotypes 1, 2, 3, 4, 5 and 6. For Gilead's Sovaldi, it is only valid for type 1, 2, 3 and 4 and must be administered simultaneously with Ribavirin. For this reason, Mavyret currently occupies 75% of the domestic hepatitis C treatment market based on UBIST last year. Sovaldi, by contrast, stood at 2%. Sovaldi had a 62% market share in 2017. Gilead's other hepatitis C drug, Harvoni, came in second with a 20% share after Mavyret last year. Epclusa (Velpatasvir + Sofosbuvir) was also received. Epclusa is also a pangenotypic treatment that can be administered regardless of genotype, a competitive drug for Mavyret. If the two drugs are registered in Korea, it is predicted that they will replace performance of Sovaldi and Harvoni. Although the market for hepatitis C drugs is decreasing in size due to the decrease in the number of patients due to effective drugs, it is attractive for pharmaceutical companies as Mavyret achieved ₩32.6 billion in outpatient prescription last year. As Gilead prepares a new drug, the domestic hepatitis C treatment market is expected to enter fierce competition again.