- LOGIN
- MemberShip
- 2025-12-18 03:04:58
- Policy
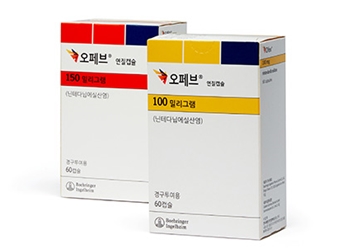
- Yungjin, Ildong’s Ofev generics enter market at half price
- by Lee, Tak-Sun Jun 26, 2025 06:07am
- A generic version of Ofev (nintedanib), a treatment for chronic fibrotic interstitial lung disease), will enter the market at half the price of the original drug. With the entry of generic drugs, the Ofev market now faces competition, just two months after the original drug was listed for reimbursement. According to industry sources on the 25th, Yungjin and Ildong Pharmacuetical will list the generic version of Ofev with reimbursement at a price much lower than the calculated price, differentiating it from the original product. Also, the original product is a capsule formulation, while the generic version is a tablet formulation. When reimbursement was approved in May, the maximum insurance price for Boehringer Ingelheim Korea's Ofev Soft Capsules 100 mg was set at KRW 29,600, and Ofev Soft Capsules 150 mg at KRW 26,220. The generic versions are much cheaper than this. Three generic drugs will be listed for reimbursement next month: Yungjin Pharmaceutical's Nintebro Tab 150 mg and Nintebro 100 Tab mg, and Ildong Pharmaceutical's Cuninta Tab 150 mg. Among them, Nintebro 100 mg has a maximum price of KRW 9,000, less than half the original Ofev Soft Capsule 100 mg (KRW 20,960). Nintebro 150 mg is KRW 15,000, and Cuninta 150mg is KRW 13,500. When considering how Ofev Soft Cap 150mg costs KRW 26,220, the generic versions cost half the original price. Being rare disease treatments, the generic versions were eligible to receive the same price as the original Ofev, but it is believed that the companies significantly lowered the price in consideration of market competitiveness. As a result, patients will now be able to purchase the same ingredient drug at a lower price. In May, after nine years since being approved in Korea, Ofev was listed for reimbursement as a treatment for chronic fibrotic interstitial lung disease. Due to the delay in reimbursement listing, the substance patent expired on January 25. This is how generic versions were able to enter only 2 months after the original drug was listed for reimbursement. Meanwhile, Yungjin and Ildong, which are launching the generic versions, are competing fiercely in the pirfenidone market, a representative treatment for idiopathic pulmonary fibrosis, which is another indication for Ofev.
- Policy
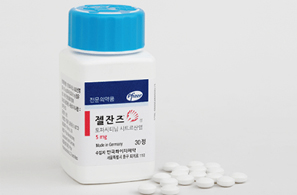
- Daewoong's high-dose generic 'Xeljanz' wins nod
- by Lee, Hye-Kyung Jun 26, 2025 06:06am
- Product photo of Daewoong Pharmaceutical's oral generic version of 'Xeljanz (tofacitinib),' which is used to treat rheumatoid arthritis, received approval. On June 23, the Ministry of Food and Drug Safety (MFDS) approved Daewoong Pharmaceutical's two dosages of 'Xeltofa Tab': 5 mg and 10 mg. The latest approval grabs attention, particularly because Daewoong Pharmaceutical has developed high-dose formulation of generic Xeljanz. Over 65 generic Xeljanz have been approved to date. However, only five items with a 10 mg high-dose formulation, including Pfizer Korea's 'Xeljanz,' Chong Kun Dang's 'Tofacell Tab,' Jeil Pharm's 'Topazan Tab,' Jeil Pharm's 'Topazan Tab,' and Alvogen Korea's 'Gencinib Tab,' are available. High-dose formulations have not been popular because the approved indications were fewer than those of low-dose ones. While the low-dose formulation holds five indications, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, polyarticular juvenile idiopathic arthritis (pJIA), and juvenile psoriatic arthritis, the high-dose formulation is only approved for ulcerative colitis, thereby limiting its therapeutic scope. Safety concerns and fewer indications have hindered high-dose generic Xeljanz approvals. However, an increasing number of pharmaceutical companies are now securing approvals for both low-dose and high-dose products. With the substance patent for Xeljanz expiring in November, Korean companies are expected to expedite getting approvals for their generics. Xeljanz holds a substance patent expiring in November 2025 and a crystal form patent expiring in November 2027. Twenty companies, initially by Chong Kun Dang, attempted to overcome these patents by filing invalidation trials. Additionally, 16 companies, including Boryung Pharmaceutical, filed passive rights scope confirmation trials to circumvent them. In January 2018, generic companies successfully circumvented the Xeljanz patent. Following another victory in an invalidation trial in November 2019, the Xeljanz crystal form patent was removed in March 2020. Generic Xeljanz items that have obtained marketing authorization can be launched after November 22, 2025, when the substance patent expires.
- Policy
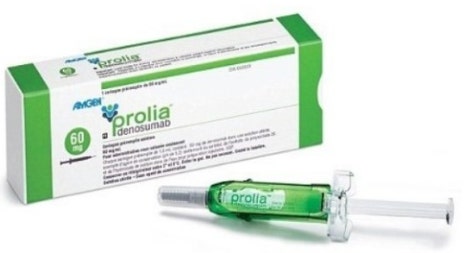
- Samsung Bioepis joins Prolia market with Obodence
- by Lee, Tak-Sun Jun 25, 2025 06:02am
- With Samsung Bioepis entering the biosimilar market for Prolia (denosumab), competition is expected to intensify among co-promoter companies Daewoong Pharmaceutical (Stoboclo, Celltrion), Hanmi Pharmaceutical (Obodence, Samsung Bioepis), and Chong Kun Dang (original Prolia, Amgen). Chong Kun Dang signed a co-promotion agreement with the original manufacturer, Amgen; Daewoong Pharmaceutical with Celltrion; and Hanmi Pharmaceutical with Samsung Bioepis, respectively. According to industry sources on the 24th, Samsung Bioepis' Prolia biosimilar Obodence Prefilled Syringe (denosumab, 60mg/1mL) will be listed for reimbursement in July. As a result, Celltrion's ‘Stoboclo Pre-filled Syringe’ and Samsung Bioepis' ‘Obodence Pre-filled Syringe’ are expected to compete fiercely in the domestic market. Park Jae-Hyun, President of Hanmi Pharmaceutical (right), and Kim Kyung-Ah, CEO of Samsung Bioepis (left), sign a copromotion agreement in March and pose for a commemorative photo. Celltrion’s Stoboclo was listed for reimbursement in March. The maximum reimbursement ceiling price for Stoboclo and Obodence is the same. Obodence’s price was set the same as the lowest price of the same ingredient drug already listed when it was added to the reimbursement list. As there are between 2 and 19 drugs of the same type, and the price of the drugs have already been adjusted to 70%, Obodence’s price was calculated at the same price as the lowest price of the same drug already listed in accordance with the calculation rules for biological drugs. The lowest price among the same-ingredient drugs was Stoboclo. Stoboclo’s price was voluntarily reduced by Celltrion from KRW 111,384 to KRW 108,290 starting in July, and Obodence’s price was also set accordingly at KRW 108,290. The original Prolia’s insurance ceiling price is KRW 123,760. Prolia is the absolute leader in the osteoporosis treatment market. According to IQVIA, Prolia recorded sales of KRW 174.9 billion in the domestic market last year. Prolia suppresses the activity of osteoclasts responsible for bone degradation, thereby preventing bone resorption while promoting an increase in bone mineral density. The drug is regarded to have improved the convenience of administration for patients, as its efficacy can be maintained for 6 months with a single injection. Daewoong Pharmaceutical and Celltrion Pharmaceutical announced in October last year that they signed an agreement for the copromotion of a Prolia biosimilar With a domestic market size worth KRW 170 billion for that single drug, large pharmaceutical companies have actively joined in the competition to become the distributors of denosumab and its biosimilars. The original Prolia has been sold jointly by Chong Kun Dang and Amgen since 2017. Daewoong Pharmaceutical stepped in as a relief pitcher for Celltrion's Stoboclo. Chang-Jae Lee, CEO of Daewoong Pharmaceutical, said after the copartnership agreement signing ceremony, “We expect that the agreement will leverage the strong sales and marketing capabilities of Daewoong Pharmaceutical and the outstanding biosimilar technology of Celltrion to dominate the osteoporosis treatment market. Furthermore, we will strive to reduce the risk of fractures and improve the quality of life for osteoporosis patients in Korea with CT-P41, in a prescription market worth KRW 170 billion.” Hanmi Pharmaceutical will co-market Samsung Bioepis' Obodence. Jae-Hyun Park, President of Hanmi Pharmaceutical, said, “Hanmi Pharmaceutical is a well-prepared partner with an established foothold in the musculoskeletal treatment market. Through the partnership, we will closely collaborate with our partner to create innovative results that promote mutual growth.”
- Policy
- Inspections target faulty packaging·labeling of drugs
- by Lee, Jeong-Hwan Jun 24, 2025 06:00am
- The Korean government and local governments will launch inspections targeting pharmaceutical companies with a history of recalls due to faulty packaging or labeling of medicinal products and illegal advertising of GLP-1 obesity drugs, such as Wegovy. For GLP-1 obesity drugs, to prevent misuse and abuse, inspections will focus on handling medical clinics and pharmacies to check for direct-to-consumer advertising of specialized medicines and whether information exceeding the scope of approved indications is being exaggerated or falsely advertised to the general public. Following the inspections, pharmaceutical companies, medical institutions, pharmacies, and wholesalers found to violate these terms will face measures such as administrative guidance or administrative penalties. On June 23, the Ministry of Food and Drug Safety (MFDS) announced that it would conduct planned joint inspection with local MFDS agencies and local governments to strengthen the safety management of medicinal products· biopharmaceutical products·Korean traditional medicines·quasi-drugs·medical devices. This second-quarter joint inspection of medical products will run until June 27. For medicinal products, manufacturers with a history of recalls due to packaging·labeling defects will be inspected, given the continuous occurrence of such recalls. Key inspection points include, ▲Whether the company has implemented follow-up measures and recurrence prevention plans submitted during previous drug recalls ▲Whether follow-up measures from self-inspections related to packaging·abeling processes have been carried out. If follow-up measures are not implemented or violations are confirmed during the inspection, necessary actions, including administrative penalties, will be taken. During the summer, concerns about misuse and abuse are rising due to illegal advertising of specialized medicines, including online posts featuring post-treatment reviews that describe GLP-1 obesity biopharmaceuticals as 'weight loss drugs' or promotional materials placed in patient waiting areas. To prevent the misuse and abuse of obesity treatments, MFDS will focus on handling medical clinics and pharmacies to inspect ▲Whether prescription drugs are being advertised to the general public through various advertising media ▲Whether exaggerated·false information exceeding the scope of approved indications is being released to an unspecified public. If violations are confirmed during the inspection, necessary actions such as administrative guidance or administrative penalties will be taken against the medical clinics, pharmacies, and, if necessary, wholesalers or pharmaceutical manufacturers. For Korean traditional medicines (Hanyak), rigorous quality control is required for high-value herbal ingredients, such as 'Nokyong' and 'Uhwang'. Therefore, manufacturers of these herbal ingredients and manufacturers of hanyak ingredients containing 'Uhwang,' such as 'Uhwangcheongsimwon,' will be inspected. Key inspection points will include ▲Appropriateness of quality control for raw materials and finished products ▲Appropriateness of raw material storage management and finished product manufacturing management. For quasi-drugs, to ensure a safe distribution environment for anti-snoring products used to reduce or suppress snoring noise, manufacturing (importing) companies will be inspected for ▲Performance of quality inspections for raw materials and finished products ▲Microbiological quality control ▲Appropriateness of hygiene management of manufacturing facilities. Additionally, inspections for false·exaggerated advertising will be conducted on sanitary product vendors frequently found with identical advertising violations on numerous online sales sites. If violations are confirmed in advertisements provided by manufacturing (importing) companies, those manufacturers (importers) will also be inspected. As instances of unapproved illegal medical devices being imported through direct overseas purchases or purchasing agencies continue to occur, companies frequently caught importing illegal medical devices at the import·customs clearance stage will be inspected. Key inspection points will include ▲Whether illegally imported medical devices are being illegally distributed ▲Whether return or disposal measures have been taken ▲Other violations of the Medical Devices Act. If inspections confirm violations, such as storing or distributing illegally imported medical devices without returning or disposing of them, necessary actions, including administrative penalties, will be taken. Meanwhile, to enhance the effectiveness of this planned joint inspection, the MFDS conducted pre-training on inspection methods for the '2025 Q2 Medical Products Sector Inspection Officer Training' (6.19.~20.), involving local MFDS agencies and local governments (cities·provinces, cities·counties·provinces).
- Policy
- Industry expects improved pricing measures by new gov't
- by Lee, Jeong-Hwan Jun 23, 2025 06:01am
- The domestic pharmaceutical industry is closely monitoring the direction of the Lee Jae-Myung administration's preferential drug pricing policy. The industry is most interested in when and how the presidential election pledges, including specific implementation plans for the new drug R&D-linked preferential drug pricing policy and the integration and advancement of the post-marketing drug price management system, will be realized. Multiple pharmaceutical companies believe that the public-private consultation body for the advancement of the drug price system, whose operation has been suspended around the presidential election, must be restarted as soon as possible in order to prompt practical discussions on the presidential campaign pledges. On the 22nd, the pharmaceutical industry expressed expectations that the Lee Jae-Myung administration's inauguration would bring significant changes to the drug price system, which will directly impact the promotion of the pharmaceutical and bio industry. The Ministry of Health and Welfare announced a health insurance operation plan during the previous administration on how it would establish various preferential pricing measures for drugs by domestic pharmaceutical companies. The previous Yoon Suk-Yeol administration's blueprint for drug price regulations was to favor drug prices or to partially defer or exempt drug price reductions of pharmaceutical companies that contributed to improving public health, enhancing the sustainability of health insurance, and developing the national economy. Additionally, the previous government promised to integrate the fragmented post-approval drug price management system into a single framework to address issues such as redundant price reductions that deter new drug development, thereby enhancing predictability for pharmaceutical companies and supporting their business operations. However, multiple pharmaceutical companies have pointed out that there are few actual cases where the previous government's plans have been implemented in practice. In other words, efforts to establish the criteria for preferential drug pricing that can promote new drug development, create jobs, and save national health insurance finances, have been stagnant for over a year. According to industry sources, the only drug price policies that have actually been implemented are regulations that favor the prices of essential medicines manufactured with domestic ingredients and the temporary suspension of the price reduction system based on external reference pricing reevaluations that were about to be implemented. Accordingly, the pharmaceutical industry is closely watching whether the new government will expedite the establishment of national economic development criteria that would allow pharmaceutical companies to receive preferential drug pricing. Given that President Lee Jae-Myung has pledged to implement a preferential policy linking new drug R&D ratios to drug prices, expand measures that take into account the pharmaceutical industry’s social contribution, and modernize the post-management of drug prices in an integrated manner, industry attention is now focused on when and how the Ministry of Health and Welfare and other relevant authorities will initiate specific reforms in the drug pricing system. A representative from a mid-sized domestic pharmaceutical company explained, “The previous administration had repeatedly announced plans to introduce new preferential drug pricing clauses and the reform of the drug pricing system, but in reality, the progress felt slow. Preferential treatment was mainly limited to essential medicines using domestic raw materials, and even then, the number of items actually benefiting from the policy was quite small.” The representative added, “While the implementation of the external reference pricing reevaluation results has been temporarily suspended due to ongoing concerns from pharmaceutical companies, significant concern remains on how it could be reinstated at any time. The key issue is how much and in what direction the new government will diverge from the previous administration's drug price system policies.” Another domestic pharmaceutical company official noted, “The adoption of a drug pricing system that provides greater compensation to pharmaceutical companies that invest heavily in new drug R&D as a presidential election pledge holds significance.” “While policies that provide price advantages to pharmaceutical companies that increase investment in new drugs and make efforts to reduce national health insurance expenditures are important, reducing or eliminating unreasonable drug price reduction mechanisms could have a more substantial impact on pharmaceutical companies.” The official added, “A drug pricing system that adequately reflects the characteristics of the domestic pharmaceutical industry and advanced post-approval drug pricing management policies must be implemented by the new government to enhance pharmaceutical companies' capacity to invest in new drugs. To establish systems that enhance the predictability of the pharmaceutical companies’ business operations, we hope that the new government will first establish a public-private consultation body.”
- Policy
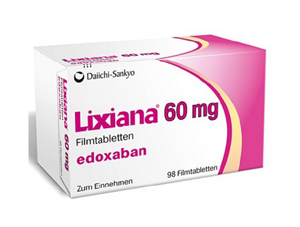
- Generic 'Lixiana' is now in 'active' development in KOR
- by Lee, Hye-Kyung Jun 23, 2025 06:01am
- Korean pharmaceutical companies are actively working on plans to launch their generic version of 'Lixiana Tab 60 mg (edoxaban tosylate hydrate),' a Direct Oral Anti-Coagulant (DOAC) with patent expiry set to November of next year. The Ministry of Food and Drug Safety (MFDS) recently approved Korean Drug Co's application for a bioequivalence trial. This trial is an open-label, randomized, single-dose oral, two-group, two-period, crossover study in healthy adults, comparing "Korea-edoxaban 60mg Tab" with Daiichi Sankyo's 'Lixiana Tab 60mg (edoxaban tosylate hydrate)'. This month, two bioequivalence test approvals have been granted for the development of generic Lixiana. Korea Arlico Pharm received approval for "Arlixaban Tab 60mg" on June 9, before the Korean Drug Co. According to the approval history of bioequivalence tests for generic Lixiana, the MFDS has approved 10 cases. Over ten pharmaceutical companies, including Boryung, Dong-A ST, HK inno.N, Samjin Pharmaceutical, Shinil Pharma, Chong Kun Dang, Kolmar Pharma, Korea Kolmar, Hutecs, and Hanmi Pharmaceutical, have already successfully avoided the patent and secured generic approvals. Seventeen Lixiana generic products are currently listed in the approval registry. Theragen Etex and Dongkwang Pharmaceutical, which filed patent invalidation suits last year, also recently received 'claim upheld' rulings. Korean companies that have successfully avoided the patent will be able to launch their generics when the substance patent expires in November 2026. Late entrants to generic development are actively working to accelerate their generic launches by filing a passive rights scope confirmation trial related to Daiichi Sankyo's pharmaceutical composition patent. Currently, HLB Pharma and Samjin Pharm have filed passive rights scope confirmation trials related to Lixiana's formulation patent in January and March, respectively. The Lixiana patents listed in the MFDS patent registry include a substance patent expiring in November 2026 and a formulation patent expiring in August 2028. Challenges to the Lixiana formulation patent began in 2020. At that time, about 10 companies, including Boryung, filed an invalidation trial aimed at the Lixiana formulation patent in July 2018. Within approximately two years, the challenging companies won the first instance. The victory was confirmed without an appeal from Daiichi Sankyo. Since December 2021, companies including Nexpharm Korea, Dong-A ST, Samsung Pharmaceutical, Shinil Pharma, Shin Poong Pharm, Ahngook Pharm, Ildong Pharmaceutical, Genuone Sciences, Union Korea Pharm, Korea Prime Pharm, Hutecs, and Handok have each obtained generic marketing authorizations. Lixiana has consistently maintained its lead in the DOAC market for a long time, with annual prescription sales exceeding KRW 100 billion. According to pharmaceutical market research firm UBIST, Lixiana's prescription sales in 2023 were KRW 105.3 billion, a 9% increase from KRW 96.7 billion in 2022. In the first half of last year alone, it recorded KRW 55.7 billion in prescription sales. Lixiana first took the market lead in 2019 and has consistently held that position. Analysis suggests that the joint selling synergy with Daewoong Pharmaceutical has positively contributed to the increase in prescription sales.
- Policy
- Handok and JW’s new imported drugs reimbursed from July
- by Lee, Tak-Sun Jun 23, 2025 06:00am
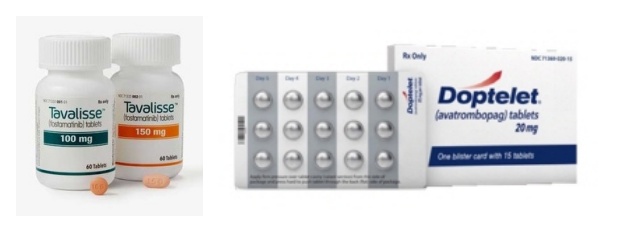
- Tavalisse (JW Pharmaceutical) and Doptelet (Handok) will both be reimbursed for ITP from next month In July, four new drugs, including an oral drug for paroxysmal nocturnal hemoglobinuria (PNH), a new biological agent for severe atopic dermatitis, and a new drug for immune thrombocytopenia (ITP), will be reimbursed by health insurance and be available with coverage for patients. On the 19th, the MOHW announced the establishment of reimbursement standards for four new drugs, including Fabhalta Cap 200 mg (Iptacopan hydrochloride monohydrate, Novartis Korea), a new drug for the treatment of PNH. The reimbursement will take effect on the 1st of next month. In addition to Fabhalta Cap 200 mg, a treatment for severe atopic dermatitis, Ebglyss Autoinjector 250 mg (lebrikizumab-lbkz, Lilly Korea), ‘Doptelet Tab 20mg (avatrombopag maleate, Handok),’ and ‘Tavalisse Tab 100·150mg (fostamatinib sodium hydrate, JW Pharmaceutical).’ Fabhalta Cap 200 mg is an oral treatment for paroxysmal nocturnal hemoglobinuria and is expected to be more convenient than the injectable drugs that have been used until now. In addition, its hemolysis control effect is expected to be better than existing C5 inhibitors (Soliris and Ultomiris). PNH is a rare condition that affects about 1.5 people per 1 million worldwide. Until now, Soliris (eculizumab) and Ultomiris (ravulizumab) have been the main C5 inhibitors used for PNH. In November last year, Empaveli (pegcetacoplan, Handok), a subcutaneous injection that binds to C3 and C3b to inhibit the complement cascade, was also approved for reimbursement. The reimbursement of the new oral drug Fabhalta is expected to improve access to treatment and offer better therapeutic effects. Ebglyss Autoinjector 250 mg is reimbursed for adult (18 years of age or older) and adolescent (12 to 17 years of age, weighing 40 kg or more) patients with chronic severe atopic dermatitis whose symptoms have persisted for three years or more. This drug is the third biological agent for atopic dermatitis, following Dupixent (dupilumab) and Adtralza (tralokinumab). Ebglyss has demonstrated clinical efficacy and safety profiles through a Phase III clinical trial and is expected to become a first-line treatment option for atopic dermatitis patients in Korea. Doptelet Tab and Tavalisse Tab will be reimbursed for the treatment of adult chronic immune thrombocytopenia (ITP) that is unresponsive to corticosteroids and immunoglobulin. ITP is an autoimmune disease in which the immune system mistakes platelets for viruses and attacks them, causing platelet counts to fall below normal levels and increasing the risk of bleeding. As a result, patients may experience easy bruising and frequent bleeding, which in severe cases can lead to cerebral hemorrhage or gastrointestinal bleeding. According to the ITP Clinical Practice Guidelines issued by the Korean Society of Hematology, steroids and immunoglobulins are recommended as first-line treatments, while splenectomy or thrombopoietin-receptor agonists (TPO-RA) are recommended as second-line treatments for chronic ITP patients. With rare disease drugs imported by domestic pharmaceutical companies such as Handok and JW Pharmaceutical now reimbursed, industry eyes are on how the drugs will be received in the market.
- Policy
- Rapid adjustment of insurance prices for rare drugs granted
- by Lee, Hye-Kyung Jun 20, 2025 06:08am
- Verorab (pictured above), for which a price adjustment has been completed in response to exchange rate fluctuations, and Ivexproglycem (pictured below) for which a price adjustment application is currently under negotiations. The insurance price ceiling adjustment term for insured drugs supplied by the Korea Orphan Drug Center (KOEDC) is expected to be significantly reduced from the initial 8-9 months to around 4 months. Previously, it took at least 8-9 months to adjust the price cap, raising concerns about supply disruptions and financial losses for the center, so the government decided to take proactive measures to resolve the issue by significantly simplifying the price adjustment process for urgently needed drugs. During a meeting with journalists from the Ministry of Food and Drug Safety’s press corps on the 17th, the KOEDC said that it is operating a consultation system to promptly adjust reimbursement price ceilings for publicly procured orphan drugs in order to ease the supply burden caused by sharp exchange rate fluctuations. The KOEDC added that it adjusted the insurance price ceiling for Verorab through the system in January. The insurance price of Verorab was KRW 63,000 before the adjustment, but after the adjustment, it was raised by 65.9% to 104,887 won. According to the center, most orphan drugs are highly dependent on overseas imports, and import prices are sensitive to exchange rate fluctuations. However, the reimbursement ceiling was fixed, so there was a structural problem where the center had to continue supplying the drugs at a loss when the exchange rate rose sharply. According to the center, the exchange rate has risen by about 20% in the last three years, and in the case of some items, the KOEDC incurred losses of more than KRW 100 million per case. This has directly impacted patients' access to treatment. When the center delays or suspends supply due to budget constraints, patients experience treatment gaps because there are no domestic alternatives for orphan drugs. As a result, patient groups and relevant experts have consistently raised the need for system improvements. Kim Ki-young, Director of KOEDC, explained, “There has been around a 20% fluctuation in exchange rates over the past 3 years. For orphan drugs distributed in the market based on the reimbursement price ceiling, the increased costs have led to losses exceeding KRW 100 million per drug. In practice, the total budget for drug purchases has decreased, which has also caused disruptions in the supply of subsequent orphan drugs.” In response, the center established an exceptional price adjustment procedure in collaboration with the Ministry of Health and Welfare, the Ministry of Food and Drug Safety, the Health Insurance Review and Assessment Service (HIRA), and the National Health Insurance Service (NHIS). This streamlined process applies exclusively to publicly procured items that meet specific criteria. It significantly shortens the price ceiling adjustment period from several months to within 10 days, thereby minimizing supply disruptions and ensuring continuity of patient care. Notable examples of the expedited adjustment procedure include Verorab, which was adjusted in January this year, and Ivexproglycem, which is currently undergoing price adjustment. Kim said, “Previously, adjustments to the reimbursement price ceiling took eight to nine months due to various procedures, which inevitably resulted in losses for the center. The MFDS requested cooperation from the MOHW to establish a procedure to correct supply instability so that adjustments can be made quickly in response to exchange rate fluctuations.” Just like with exchange rate increases, price adjustments are also promptly made in the event of a decrease. Director Kim said, “If an exchange rate drop results in a surplus, the revised amount can be reflected starting on the first day of the following month. This new cooperative framework enables more reasonable adjustments and contributes to the stability and sustainability of public procurement by government agencies.” In addition, KOEDC is pursuing various policies to improve access to orphan drugs. Key initiatives include operating patient assistance programs in partnership with pharmaceutical companies and providing free or cost-based supply of drugs for which price negotiations are delayed. Also, the center is working to establish overseas supply chains to introduce medications for self-treatment and is running an early distribution program for pediatric orphan disease treatments.
- Policy
- SGLT2 Jardiance likely to receive expanded reimb for CKD
- by Lee, Tak-Sun Jun 20, 2025 06:06am
- Product photo of JardianceThe SGLT-2 inhibitor Jardiance (empagliflozin, Boehringer Ingelheim) is expected to be reimbursed for the treatment of chronic kidney disease (CKD). This drug is currently reimbursed for the treatment of diabetes and chronic heart failure. If expanded reimbursement is approved for CKD, the volume of usage is likely to be increased. Furthermore, its market presence is expected to be strengthened in the SGLT-2 inhibitors market. According to industry sources on June 19, the agenda related to Jardiance's expanded reimbursement to include CKD passed the Drug Reimbursement Evaluation Committee (DREC) held on the 12th. Once it undergoes the National Health Insurance Service's simplified negotiation process and reports to the Health Insurance Policy Deliberation Committee, Jardiance is anticipated to be reimbursed for treating CKD. Since the company already completed drug price estimation process, reimbursement may be approved as early as July 1. It has been reported that Jardiance's expanded reimbursement for CKD was approved for the pre-drug price reduction program for expanded usage scope drugs. This program is designed to quickly enhance patient access to treatments by omitting the cost-effectiveness evaluation and applying a pre-reduction rate table, with a maximum 5% reduction in the ceiling cap, considering the estimated additional claim amounts due to the expanded reimbursement criteria. If a drug's ceiling price is adjusted via this program, its company will only have to undergo negotiations related to supply requirements, such as those required for other reimbursed medicines. Jardiance's company applied to the Health Insurance Review & Assessment Service (HIRA) for expanded reimbursement for Jardiance's CKD indication in the first half of last year. Jardiance was the first SGLT-2 inhibitor to file. Jardiance Tab 10mg is currently indicated for the treatment of 1. Type 2 diabetes mellitus, 2. chronic heart failure, and 3. chronic kidney disease. Jardiance Tab, which was listed for reimbursement as a diabetes treatment in 2017, has also been reimbursed for chronic heart failure since February 2024. Since January this year, the reimbursement criteria have been expanded, allowing patients with heart failure with preserved ejection fraction (HFpEF) who have symptoms and signs of heart failure and a left ventricular ejection fraction exceeding 40% to receive national health insurance benefits. Currently, the only SGLT-2 drugs covered for diabetes and chronic heart failure patients, besides Jardiance, are Forxiga (dapagliflozin) and HK inno.N's DapaN Tab, which transferred Forxiga's indications. However, there is currently no drug reimbursable for CKD. If the reimbursement expansion procedure for Jardiance is completed, it is expected to be the only SGLT-2 inhibitor with coverage for CKD reimbursement. The efficacy of Jardiance has been demonstrated through clinical trials, showing a statistically significant 28% relative risk reduction in the progression of kidney disease or cardiovascular death compared to placebo. The clinical trials that Jardiance significantly reduced a relative risk in the progression of kidney disease or cardiovascular death compared to placebo by 28%. The expanded scope of use will provide advantage in competition with generic drugs. Jardiance's substance patent expires in October this year. Therefore, tens of domestic generic drugs are likely to enter the market. Jardiance, jointly sold by Boehringer Ingelheim and Yuhan, recorded outpatient prescription sales of KRW 66.3 billion last year, according to UBIST.
- Policy
- Growing role of the Korea Orphan & Essential Drug Center
- by Lee, Hye-Kyung Jun 19, 2025 06:03am
- "The emergency import of essential medicines through the Korea Orphan & Essential Drug Center (KOEDC) will be expanded, and support for pharmaceutical companies producing domestic products will be planned." President Lee Jae-myung made this pledge on his Facebook page on May 28, during his campaign for the presidency. President Lee emphasized the importance of the KOEDC, stating that he would strengthen national guarantees for the treatment of rare and intractable diseases. However, the KOEDC is a Ministry of Food and Drug Safety (MFDS)-affiliated organization with a fixed quota of only 30 personnel. KOEDC is responsible for the supply of rare and national essential medicines, establishing a stable supply base for national essential medicines, and supporting R&D. The center is currently understaffed. Kim Young-rim, CEO of Korea Orphan & Essential Drug Center (KOEDC)Kim Young-rim, CEO of KOEDC, stated at a briefing for journalists covering the MFDS on June 17, "The global supply chain has become increasingly important after COVID-19, and the role of the center is also growing." Kim added, "As a small organization with only 30 personnel, where individual roles and responsibilities are significant, it is necessary to devise ways to perform duties efficiently." In particular, the emergency import of essential medicines mentioned by President Lee is part of the national essential drug supply management plans implemented by the KOEDC. The KOEDC plays a crucial role in managing emergency imports of medicines that could be subject to potential supply shortages from overseas, domestically produced products under contract manufacturing, and emergency use authorizations for items deemed necessary to address public health crises. Kim stated, "The KOEDC is a specialized organization responsible for the stable supply of rare and essential medicines in Korea. This year, we plan to actively identify and promote tasks in line with the new government's initiatives to strengthen our role in ensuring a stable supply of rare and essential medicines." Regarding national essential medicines, strengthening on-site supply and demand monitoring, as well as emergency importation of drugs, is one of the main tasks for this year. The goal is to enhance management analysis of discontinued drugs, thereby shortening the designation period for emergency imported drugs and their subsequent domestic entry. Additionally, it aims to prioritize the classification of emergency imported drugs to facilitate appropriate inventory management. The KOEDC's role also includes planning the details for stable supply, such as identifying annual supply and demand plans for drugs requested by government ministries and expanding procurement sources. In addition to national essential medicines, the KOEDC also announced plans for rare diseases this year. Kim said, "For items that have been labeled as essential in areas where no existing treatments were available and have been designated as MFDS GIFT (Global Innovative products Fast Track) and approved, we will temporarily supply them during the domestic supply gap period before drug price negotiations, contributing to increased patient access." They are also planning expedited reimbursement applications for pediatric drugs such as 'Glucagen hypo kit,' 'Baqsimi Nasal Spray,' and 'Rapamune Syrup.' KOEDC is exploring ways to reduce patient burden by converting candidates to tax-exempt status after reviewing relevant provisions. The KOEDC has been conducting the "Research on Establishing a Domestic Stable Supply Base for National Essential Medicines" since 2022 to establish a domestic self-sufficiency base for national essential medicines in response to supply chain crises. Through Phase 1 of the project, conducted from 2022 to 2023, a domestic production base for two finished pharmaceutical products and three active pharmaceutical ingredients (APIs) has been established. Ahn Myung-soo, a division head at the KOEDC, stated, "Myung In Pharm used to import benserazide raw material from China, but we have developed it domestically and completed its DMF registration with MFDS." Ahn added, "While commercialization is a decision for the pharmaceutical company due to drug pricing issues, its significance lies in being registered in the DMF so that it can be used at any time." Furthermore, Korus Pharm's amiodarone injection received approval for export. With additional processing, a push for technology transfer, and further R&D investment, it could become commercialization-ready. Since last year, Phase 2 of the project has been underway, which includes the domestic development of both the API and finished drug for acetaminophen. Ahn added, "We have succeeded in achieving self-sufficiency through the domestic development of the API and finished pharmaceutical product for acetaminophen. A product approval application will soon be submitted to MFDS," and added, "This means that domestic self-sufficiency is possible if there are shortages, regardless of the global supply chain." In this regard, Kim stated, "There are limitations to securing a stable supply network through projects funded by the KOEDC." Kim added, "Budget and personnel support are necessary for these projects to be sustainable within the KOEDC's scope. To achieve this, we plan to work towards this continuously."