- LOGIN
- MemberShip
- 2025-12-23 20:54:48
- Policy
- The fatality rate of Omicron is low
- by Lee, Jeong-Hwan Mar 08, 2022 06:08am
- The quarantine authorities announced a full-fledged easing of distancing, saying that it is becoming clearer that the COVID-19 Omicron mutation has a lower severity and fatality rate than the delta mutation. From the 5th to the 20th, the 22 o'clock operating hours limit, which will be applied to groups 1, 2, and 3, will be increased by 1 hour to 23:00, but the next adjustment of distance will be eased. In particular, the quarantine authorities urged government agencies to actively participate in blood donation and spread the voluntary participation of the people, citing the continued stage of interest in domestic blood reserves of more than 3 days and less than 5 days. On the 4th, Lee Ki-il, the first controller of the Central Disaster and Safety Countermeasure Headquarters, made the remarks at a regular COVID-19 briefing. The quarantine authorities explained that it is not enough to keep a recently relaxed distance to resolve the difficulties of self-employed and small business owners. Considering that the quarantine system is being reorganized around the management of high-risk groups, the intention is to further ease distancing. Accordingly, the quarantine authorities will adjust the distance measure, which was originally scheduled to be applied by the 13th. The quarantine authorities analyzed that unlike Delta, it is becoming increasingly clear that Omicron has a low severity and fatality rate, and that the effectiveness and efficiency of distancing are decreasing due to its high propagation power. Based on this, the 22-hour operation time limit, which is applied to some facilities of groups 1, 2, 3 and other groups, will be eased by 1 hour from the 5th to the 20th until 23:00. Private gatherings maintain standards that allow up to six people nationwide regardless of whether they are vaccinated or not. The quarantine authorities plan to lower the level of distance in earnest after this easing. Pre-recommendations are also implemented for all inpatients with quasi-severe and moderate symptoms. As of the 4th, 731 patients with COVID-19 quasi-severe and moderate hospitalization who have passed 10 days from the date of sample collection are advised in advance to move to general beds. If the medical staff determines that additional quarantine treatment is necessary and submits explanatory data on all recommendations, if the need for additional quarantine is recognized through consultation, they can continue to receive treatment in the same hospital room without all or lost. If treatment is needed for an underlying disease, pt will move to a general bed and continue to receive treatment. The quarantine authorities also requested blood donation to stabilize blood supply and demand. Recently, the stage of interest in blood retention of more than 3 days and less than 5 days continues. In order to stabilize blood supply and demand, government agencies will take the initiative for a month in March and conduct group blood donations through a "Continuing Blood Donation Event." More than 1,600 employees from 31 organizations, including the Ministry of Employment and Labor, the MOHW, the Ministry of National Defense, the Maritime Police Agency, and the Ministry of Public Administration and Security, will participate in the event. In addition, the quarantine authorities asked the public to participate in blood donation at a blood donation house or a blood donation cafe close to them.
- Policy
- DREC rejects reimb of neurofibromatosis drug ‘Koselugo'
- by Lee, Tak-Sun Mar 08, 2022 06:08am
- The new neurofibromatosis drug ‘Koselugo (selumetinib),’ failed to receive reimbursement benefits in Korea. The Health Insurance Review and Assessment Service decided the drug as a ‘non-benefit' after deliberation on the adequacy of providing medical benefit for the drug at the 3rd Drug Reimbursement Evaluation Committee that was held this year. The drug, which is sold by AstraZeneca Korea, is used to treat neurofibromatosis type 1 (NF1) with symptomatic, inoperable plexiform neurofibromas in pediatric patients over the age of 3. In the past, patients had to rely on symptomatic treatment due to the lack of appropriate treatment options. This was why expectations were high for Koselugo as the new treatment option in an area with a dire unmet need. Around half of the neurofibromatosis type 1 patients have plexiform neurofibroma (PN), and it may appear anywhere in the body. Depending on the position and size, it may limit the range of motion or bring pain or external deformity in patients, The internally developed tumors may pressure the internal organs. Most rumors are positive and grow relatively slow, but some progress into malignant tumors and can lead to an increased risk of developing breast cancer in women. The prevalence is about 1 in 3,000. The drug was approved by the MFDS in May last year based on the Phase II SPRINT study. In the study, 68% of the patients who were administered Koselugo saw a decrease in tumor volume by over 20% and achieved its primary efficacy endpoint, the objective response rate (ORR). Also, 82% of the patients who showed partial response showed saw a duration in their response of over 12 months. Compared to how only half of the untreated patients experienced disease progression after 1.5 years, only 15% of the patient who used Koselugo experienced disease progression in 3 years. As the drug costs ₩200 million a year, the demand for its reimbursement had been quite strong, but the company was unable to even pass DREC deliberations, the 1st gate to reimbursement in Korea.
- Policy
- Brukinsa was approved using phase 2 data on MCL indications
- by Lee, Hye-Kyung Mar 04, 2022 05:57am
- Brukinsa 80mg (Zanubrutinib), the first new drug in Korea by Chinese pharmaceutical company Beijing, has secured MCL indications only with therapeutic clinical trial data (phase 2). This is because there was an expert opinion that according to Article 7, No. 6 of the Regulations on Item Permission, Report, and Review of Medicines, it could replace therapeutic confirmation clinical trial data. According to the minutes of the Central Pharmaceutical Review Committee on January 21, released by the MFDS on the 3rd, an advisory review on the validity of the requirements for submitting approval data related to rare drugs for MCL treatment was conducted. At the meeting, it was concluded that "MCL, which has received more than one treatment before," among Brukinsa indications, would be replaced with therapeutic confirmation clinical data as therapeutic search clinical data. With alternative treatments approved in various countries, it is practically difficult to recruit patients who have failed the first phase of MCL treatment, a rare disease, with the annual number of patients around 100, and conduct phase 3. Brukinsa is a second-line treatment for MCL, and there is no phase 3 clinical trial currently underway, and phase 3 clinical trial is underway as a first-line treatment. There were no ongoing clinical trials in Korea. Members of the Central Pharmaceutical Affairs Council said, "There is little data on comparative clinical trials in the literature, and it is a disease that is difficult to conduct research due to a rare disease." They said, "It seems practically difficult to recruit patients who have failed the first phase of treatment and proceed with phase 3 clinical trials." There was an opinion that phase 2 was more effective than the previous treatment and showed a stable aspect in terms of side effects. Some said that it is necessary to evaluate side effects and monitor safety and effectiveness-related data based on MCL indications after using drugs according to the results of phase 3 clinical trials as a first-line treatment. Brukinsa obtained permission from the MFDS on the 24th. It was recognized for its efficacy and effectiveness ▲ in WM adult patients who had received more than one treatment before ▲ in MCL adult patients who have received more than one treatment before.
- Policy
- Janumet's salt change consigned drug have been approved for
- by Lee, Hye-Kyung Mar 04, 2022 05:57am
- A large number of Janumet's salt change combination, which is commissioned and manufactured by Daewon Pharmaceutical, have been approved for items. The MFDS applied for a diabetes complex by Jin Yang, Young Poong, Withus, and Neo Bio Korea Pharm on the 2nd. 8 items of Sitaglipin HCl+Metformin and Colloidal Silicon Dioxide have been approved. This drug is an aid to diet and exercise therapy to improve blood sugar control in patients with type 2 diabetes, and Daewon Pharmaceutical developed last year as a salt change to escape the original MSD Janumet (Sitagliptin HCl+Metformin) general for exclusive. Since Daewon Pharmaceutical obtained permission on December 30 last year, 25 pharmaceutical companies have obtained permission for 73 consigned products with the same ingredients. Janumet's material patent expires on September 2, 2023, and the composition patent expires on June 1, 2024. Hanmi Pharmaceutical and Chong Kun Dang obtained the same Sitagliptin+Metformin HCl as Janumet, and Kyung Dong, Korea Prime Pharm, Yuyu Pharma, Yongjin, SCD Pharm, Dasan, Jeil, and Samjin for generic. Competition in the diabetes complex market is expected to intensify next year as Daewon's consignment product is released.
- Policy
- Ahn Cheol Soo, promised for Vaccine Sovereignty
- by Lee, Jeong-Hwan Mar 03, 2022 06:00am
- Like other party candidates, the presidential candidate of the People's Party Ahn Cheol Soo also put securing vaccine sovereignty and fostering the pharmaceutical bio-industry at the forefront of health care pledges. It also announced specific pledges such as half-price hair loss drugs, free vaccination against rotavirus in newborns, support for vaccination against HPV, and strengthening support for essential medical care. This is the result of examining health and welfare pledges in the policy pledge book released by candidate Ahn Cheol Soo on the 28th. Candidate Ahn, also promised to "take a leap forward as a vaccine sovereign state" through national investment in the bio industry. With the will not to repeat the current government's policy practice, which caused public confusion and anxiety due to the failure to secure the COVID-19 vaccine early, the government intends to implement policies to prepare for infectious diseases that will occur periodically in the future. Specifically, candidate Ahn drastically improved unreasonable regulations on vaccine development, creating an ecosystem for the vaccine industry that is good for business, and strengthening support for clinical trials. He also revealed his vision to foster the vaccine industry into our future industry by discovering and actively supporting companies with super-gap technologies in the pharmaceutical bio sector. He also specified the half-price hair loss pledge he promised earlier Candidate Ahn explained that the application of health insurance increases the financial burden because there is currently little difference in price between the original and generic hair loss drugs. He directly criticized Democratic Party candidate Lee Jae-myung's pledge to pay health insurance for hair loss. He declared that he would reduce the burden of hair loss and secure the soundness of health insurance finances by lowering the price of generic hair loss drug to 600 to 800 won. He said he would introduce incentives to revitalize generics of hair loss and adjust them with little financial input, including them in preliminary benefits, if necessary. It pledged to significantly expand R&D support for the health industry for hair loss drugs. The neonatal rota vaccine is a household burden with an inoculation cost of about 300,000 won, and the government plans to support all newborns, including the national vaccination. Like the United States, the United Kingdom, and Germany, the government and local governments provide full support for vaccination costs. He plans to expand the number of free HPV vaccines that cause cervical cancer than now. Currently, the free vaccination standard for girls under the age of 12 will be greatly expanded to men and women under the age of 26, while women under the age of 45 will be provided with vaccination fees if desired. The government-supported vaccine will be changed from Cervarix and Gardasil, the current 2-valent and 4-valent vaccines, to Gardasil 9, the 9-valent vaccine, to expand the scope of prevention. It also included policies to reduce the medical gap between regions by strengthening support for essential medical fields Candidate Ahn expressed concern that the proportion of surgeons, pediatrics, and obstetrics and gynecologists among all doctors is on the decline, and that there may be no supply due to insufficient number of majors. As a result, the government will provide indirect expenses such as wages and education expenses for training in essential medical subjects, improve the number of unpopular subjects, and take incentives to eliminate avoidance factors such as resolving disputes over medical accidents. The project to support vulnerable delivery areas will be expanded to obstetrics and gynecology medical institutions to support facilities, equipment, operating expenses, and labor costs of delivery medical institutions, and a mandatory placement system for obstetricians with more than 200 beds will be introduced.
- Policy
- To establish a pharmaceutical bio-innovation committee
- by Lee, Jeong-Hwan Mar 03, 2022 06:00am
- Yoon Suk-youl, presidential candidate for the presidential election, pledged to establish a "pharmaceutical bio-innovation committee" directly under the Prime Minister, while creating an ecosystem that can establish sovereignty in pharmaceutical bio and foster key talents and jobs in the pharmaceutical bio industry. It is said that it will overhaul the COVID-19 response system within 100 days immediately after taking office and implement the national responsibility system for vaccine side effects. The pledge also included strengthening access to medicines for patients by diversifying the rapid benefit registration system for drugs with severe rare diseases such as ultra-high-priced anticancer drugs compared to the previous one. On the 24th, Candidate Yoon Suk-youl announced a policy pledge for the power of the people in the 20th presidential election. Candidate Yoon said he would use the health care and pharmaceutical bio-industry as a new path to establishing health security and creating national wealth. He specified in his pledge the establishment of a "pharmaceutical bio-innovation committee" directly under the Prime Minister in line with the establishment of a pharmaceutical bio-control tower directly under the Blue House demanded by the KRPIA. In addition, the vision is to support national R&D to establish vaccine sovereignty and global vaccine hubs and to establish an environment to expand talent and jobs in the pharmaceutical bio industry. ◆ Reorganization of the Infectious Disease Response System = Yoon said the current government failed to respond to COVID-19. They say that they have failed to establish a proper treatment system while causing unnecessary economic damage and prolonging damage to small business owners and self-employed people by keeping distance without principles. In response, candidate Yoon plans to completely reorganize the COVID-19 response system within 100 days. The pledges include scientific and data-based quarantine measures, reorganization of the manual for responding to new infectious diseases, and conversion of public medical institutions to hospitals dedicated to COVID-19. It also declared that it will open the era of biohealth and become a vaccine and treatment powerhouse. He criticized that six domestic vaccines are currently undergoing clinical trials, but have nothing to do with government support. It is pointed out that the SK Bioscience vaccine, which is in phase 3 of clinical trials, is also being developed with technical cooperation and the CEPI support at the University of Washington in the United States. He promised to expand the overall national R&D, including high-speed vaccine development and manufacturing technology, post-corona vaccines and treatments, essential vaccines, and digital quarantine. The government's responsibility system for essential medical care and the national responsibility system for side effects of COVID-19 vaccination were included in the pledge. Under the public policy fee system, policy fees added compared to usual times are paid to doctors and nurses in emergency situations such as COVID-19, and public policy fees are also paid to severe trauma centers, delivery rooms, and newborn rooms. ◆ National Health Protection = Candidate Yoon also pledged to expand support for disaster medical expenses, ease the burden of national nursing expenses, and introduce a rapid registration system for expensive anticancer drugs and new drugs for severe and rare diseases. Among them, the rapid registration system of expensive anticancer drugs and new drugs for severe and rare diseases is a policy proposal that the KRPIA strongly appealed to when it met with party leader Lee Joon-seok. Candidate Yoon said that it takes about two years to pay health insurance benefits for new drugs with severe rare diseases such as cancer, and patients are paying high prices and administering new drugs. RSA also pointed out that it was applied only to 41 drugs, and 32 of them were concentrated on anticancer drugs. Candidate Yoon promised to shorten the health insurance registration process for anticancer drugs and treatments for severe rare diseases. It also said it will use RSA to reduce drug price negotiations and the burden on patients and insurers. In order to secure insufficient emergency and essential medical care and medical personnel in the region, the government will strengthen the publicity of local national university hospitals and upper-level general hospitals and expand them to cities and provinces without upper-level general hospitals.
- Policy
- Negotiations with NHIS on Actemra have been completed
- by Lee, Tak-Sun Mar 02, 2022 05:54am
- Actemra (Tocilizumab), a treatment for rheumatoid arthritis by JW Pharma, has been officially recognized as a treatment for COVID-19 in Korea. The drug, which has been used in patients with severe COVID-19 for purposes other than permission, will also be covered by health insurance benefits from March. On the 24th, the MOHW announced that Actemra is preparing to revise the regulations so that benefits can be applied as a treatment for COVID-19. Earlier, the NHIS negotiated with JW Pharma, a domestic importer and seller, to apply benefits. Through this, it is possible to apply benefits to severely ill patients with COVID-19. According to the revision of the MOHW, medical care benefits are recognized when administered to COVID-19 patients aged 2 or older beyond the scope of the existing permits of Actemra (excluding SC injections). "If a patient is admitted to an intensive care unit or a hospital room within 48 hours and needs respiratory treatment of HFNC (High Flow Nasal Canula) or higher," alternatively, it corresponds to "the case of administration to patients who rapidly deteriorate to a state in which respiratory treatment above High Flow Nasal Cannula (HFNC) is required even though they have been treated with steroid therapy and low flow oxygen therapy." Full support is provided through health insurance without the patient's copayment. Actemra, unlike in Korea, is already used as a treatment for COVID-19 abroad. The U.S. FDA decided on Actemra as a treatment for COVID-19 in June last year. The European Commission also approved it as a treatment for COVID-19 in December last year. Recently, the WHO also added Actemra to the Pre-qualification Review List for COVID-19 Treatment for reference by middle and low-income countries that have difficulty in independent drug screening. It has been used as an offline in Korea, but the problem is that the patient had to pay the full price of the drug because it was not paid. Other COVID-19 treatments approved in Korea are purchased entirely by the state and have no Pt's payment. Therefore, the medical community also requested the purchase of Actemra to the state in terms of equity. Actemra achieved 20 billion won in domestic sales last year, up 24.9% year-on-year. Currently, Actemra 80mg's price is 134,263 won, Actemra 200mg's price is 306,166 won, and Actemra 400mg's price is 537,060 won, while SC (subcutaneous injection) product is 345,682 won. Until now, patients have paid all the drug prices, but if health insurance benefits are applied, the patient will not be burdened with the drug prices. Actemra is also pushing for a change in permission to expand manufacturing sources limited to Japan due to the recent shortage of supply. It is also known that EUA plans as a treatment for COVID-19 are being considered. Meanwhile, JW Pharma also announced on the 25th that Actemra's benefit range will be expanded for the purpose of treating COVID-19 (COVID-19) in accordance with the revision announced by the MOHW. The company explained that the new revision, which will take effect on March 1, has changed its standards to reflect the status of overseas permits such as FDA EUA, clinical research literature, and related academic opinions. An official from JW Pharma said, "Considering the recent surge in confirmed cases of Omikron mutant virus, we have been closely discussing with health authorities to expand the benefits of Actemra. The EUA is also quickly proceeding with the MFDS." Actemra is an antibody treatment that treats diseases such as rheumatoid arthritis and pediatric idiopathic arthritis by inhibiting the binding of IL-6 and its receptors, a protein that causes inflammation in the body. It has been shown that it effectively lowers the mortality rate of severely ill and critically ill patients with COVID-19 and reduces hospitalization time in global clinical trials, a complication of excessive immune responses. JW Pharma acquired Actemra's domestic development and exclusive sales authority from Chugai under Roche Group in 2009 and has since been selling it since 2013 through phase 3 clinical trials for rheumatoid arthritis patients.
- Policy
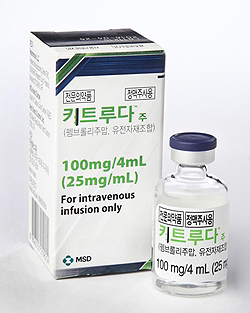
- Keytruda is reimbursed as first-line at ₩2,107,642
- by Kim, Jung-Ju Feb 28, 2022 05:54am
- The insurance price of MDS Korea’s immuno-oncology drug Keytruda(pembrolizumab) inj. that is used for non-small-cell lung cancer and Hodgkin lymphoma will drop 25.6% with its reimbursement extended from the second-line to the first-line starting next month, Astellas Korea’s Xospata 40mg (gilteritinib), as well as Novartis Korea’s Lutathera Inj. that was supplied in Korea as an urgently requested drug will also be individually listed for reimbursement as an expenditure-cap type RSA after reaching an agreement with the National Health Insurance Service. The Ministry of Health and Welfare announced on the afternoon of the 25th that it had deliberated and passed the ‘amendment to the drug benefit list and the maximum ceiling price table (draft)’ that contains the changes above as an agenda at the 5th Health Insurance Policy Deliberation Committee. ◆Keytruda(pembrolizumab) = The immuno-oncology drug Keytruda, which is indicated for the treatment of non-small-cell lung cancer and Hodgkin lymphoma, was listed as second-line treatment in August 2017, and then received an indication extension in February 2018. The company had applied for the reimbursement expansion to the Health Insurance Review and Assessment Service in October 2019 and received deliberation from the Cancer Disease Deliberation Committee on April, August, November 2020, and on May 7th last year. In January of this year, HIRA’s Pharmaceutical Reimbursement Evaluation Committee deliberated and passed Keytruda’s reimbursement expansion agenda. At the time, the PBAC judged that the ICER threshold (economic evaluation result) of Keytruda in NSCLC as monotherapy and as combination therapy in squamous and non-squamous NSCLC was appropriate, and that it was cost-effective as it costs less than its alternative brentuximab vedotin in Hodgkin's lymphoma. Also, the committee considered that the drug was listed for reimbursement in all A7 countries. The company afterward reached a final agreement with the NHIS under the Ministry of Health and Welfare’s negotiation order on the RSA drug price and expected claims amount by mid-February. In the drug pricing negotiations, the company made a refund-type RSA agreement in which the company refunds a specific proportion of the claims amount and a proportion of the amount that exceeds the expected claims cap to the government. Both parties have made an agreement to set the price at ₩2,107,642, a 25.6% discount from the current cap of ₩2,833,278 in consideration of the expanded scope of use, the standard for price cap adjustment, and its fiscal impact on insurance finances. With the refund-type RSA applied, the NHIS expects the actual fiscal spending on the drug to be less than the expected claims amount.. ◆Xospata Tab40mg (gilteritinib fumarate) = Xospata, which was approved for the treatment of acute myeloid leukemia, applied for reimbursement listing in November 2020 after receiving marketing authorization in March 2020. After deliberation by CDDC in February and PBAC in September, HIRA judged that reimbursement was appropriate for the drug. At the time, HIRA considered the fact that the drug is recommended in adult patients with FLT3-positive AML in clinical guidelines and that its cost-effectiveness also meets the pharmacoeconomic evaluation data exemption drug (PE exemption drug) conditions, and is listed in 5 of the 7 A7 countries – the US, UK, Germany, Italy, and Japan. Relevant societies have also judged that the drug improved convenience in intake as an oral formulation and may become a new treatment alternative for the disease. After passing HIRA review, the company completed drug pricing negotiations with the NHIS as a refund-type RSA. In consideration of its price abroad and fiscal impact, the two parties agreed to set the price at ₩214,100 per vial. ◆Lutathera inj.(lutetium Lu 177 dotatate) = Novartis Korea’s Lutathera Inj is a radiolabeled somatostatin analog indicated for the treatment of gastroenteropancreatic neuroendocrine tumors. The drug, which was approved in July 2020, was designated an urgently requested drug when it was first supplied in November 2019 without reimbursement and reimbursed from March 2020 ever since. In November 2020, the company applied for its formal reimbursement listing to HIRA, and the authorities deliberated the case with the CDDC in April 2021 and with DREC in November of the same year. At the time, HIRA considered the fact that the drug was recommended for the treatment of patients whose condition progressed after the current standard-of-care sandostatin lar (octreotide) in clinical guidelines (clinical efficacy aspect) and that the drug met the PE exemption criteria by applying at a price lower than the lowest A7 price(cost-effectiveness aspect), and that the drug is listed in 4 of the 7 A7 countries – the US, France, Japan, and Italy. After passing HIRA review, the company completed drug pricing negotiations with the NHIS as a refund-type RSA at ₩22,104,660 per vial.
- Policy
- BeiGene's Brukinsa has been approved in Korea
- by Lee, Hye-Kyung Feb 28, 2022 05:54am
- Brukininsa 80mg (Zanubrutinib), a BTK inhibitor from Chinese pharmaceutical company BeiGene, has obtained an item license in Korea. It is the second new drug approved by a Chinese pharmaceutical company after Antengene's Xpovio 20mg (Selinexor) in July last year. On the 24th, the MFDS approved Brukinsa, a new drug for treating blood cancer in BeiGene. This drug was recognized for its efficacy and effectiveness▲ in monotherapy in adult patients with mantle cell lymphoma (MCL) who had received more than one treatment before and▲ monotherapy in WM adult patients who had received more than one treatment before. Brukinsa is a Brutons Tyrosine Kinase (BTK) inhibitor that inhibits the survival and spread of malignant B cells by blocking BTK protein, a signaling molecule that affects the survival and development of B cells. Among the drugs licensed in Korea as BTK inhibitors are Janssen's Imbruvica 140mg, AstraZeneca's Calquence 100mg, and Ono's Velexbru 80mg. Brukinsa was quickly approved by the U.S. Food and Drug Administration (FDA) as an MCL treatment in November 2019. At that time, it became famous as the first new drug to be approved by the FDA based on clinical trials in China. It then expanded its indications in September last year when it was approved by the FDA as a treatment for WM patients. Clinical trials are currently underway for CLL, MZL, and FL patients. A new drug approved by a Chinese pharmaceutical company in Korea was first released in July last year. Antengene's Xpovio 20mg (Selinexor) has been approved in Korea for anti-cancer drug which is administered to treat refractory multiple myeloma and refractory giant B-cell lymphoma.
- Policy
- President Moon said, "WHO selects Korea as a vaccine hub"
- by Kang, Shin-Kook Feb 28, 2022 05:54am
- President Moon Jae In evaluated that the government's efforts to become a vaccine hub country have paid off, saying that Korea has been exclusively selected as the "WHO Global Bio Human Resources Development Hub." President Moon posted on SNS on the 24th and said, "This is the result of being internationally recognized for Korea's biopharmaceutical production capacity and excellence in educational infrastructure." He said, "It is the evaluation of the international community that the Korean government is taking the lead in overcoming the World Health crisis by strengthening vaccine cooperation with various countries and organizations, including Hanmi global vaccine partnership." President Moon said, "Korea has become a hub country for education and training and training to increase vaccine capabilities of developing countries. We will actively share our experiences and know-how with the international community, self-sufficiency of vaccines, and resolve vaccine inequality." "It's another opportunity for us. The world's top five vaccine powers and bio-leading countries set by the Korean government are coming to reality, he said. "It will provide young people with opportunities to form world-class education and human networks, and increase the awareness and reliability of our bio companies to help hub overseas exports and vaccine production." President Moon said, "South Korea is successful based on innovation capabilities and human investment," adding, "It is constantly advancing from a country that receives aid, from developing countries to developed countries, from pursuing countries to leading countries." The WHO announced on the 23rd that it has exclusively selected South Korea as "the WHO Global Bio Human Resources Development Hub." WHO Human Resources Development Hub refers to a central institution that provides education and training for vaccine and biopharmaceutical production processes to self-sufficiency vaccines in middle and low-income countries. In response, Minister of Health and Welfare Kwon Deok-cheol said, "The fact that Korea was designated as a hub for fostering WHO manpower is to expect leadership for global health and safety by overcoming COVID-19 and responding to the next pandemic and utilizing domestic capabilities." In response to expectations, we will develop Korea into a global bio-human resource training mecca in the future, he said.