- LOGIN
- MemberShip
- 2026-03-10 07:04:58
- Policy
- Pharmaceutical post-market management system to be enhanced
- by Jung, Heung-Jun Nov 14, 2025 06:11am
- The National Health Insurance Service (NHIS) plans to revise its drug price negotiation and post-market management systems next year, with a focus on stable pharmaceutical supply. The NHIS will actively enforce penalties stipulated in its regulations if pharmaceutical companies discontinue drug supply without notification in violation of agreements reached during price negotiations. This is a response to criticism raised during this year's parliamentary inspection that the agency was not adequately managing the supply obligation despite having the 'Drug Price-Reimbursement Agreement.' Se-rim Oh, Head of the Negotiation and Post-Management Unit at the Department of Drug Management.On November 11, the NHIS held a 'Drug Price Negotiation and Post-Market Management System Briefing' at its headquarters in Wonju, sharing the direction for management reinforcement with industry stakeholders for next year. The NHIS emphasized that if a company must inevitably suspend supply in violation of the agreement, it must formulate patient protection measures and consult with the NHIS beforehand. Se-rim Oh, Head of the Negotiation and Post-Management Unit at the Department of Drug Management, said, "The NHIS plans to strengthen management focusing on supply issues. If a company withdraws approval and suspends supply, it must ask pharmaceutical associations and formulate patient protection measures." Starting next year, the NHIS will actively consider imposing penalties on companies that violate their supply obligations, based on a fixed formula. The current formula sets the penalty amount per day of violation as: "(Previous Year's Annual Claim Amount of the Violating Drug) X 1/n X1/365X50%." Furthermore, companies will be required to submit data to the NHIS within 40 days of the end of each quarter to verify the fulfillment of their supply obligation (including monthly production volume, import volume, requested supply volume, and actual supply volume). The existing clause requires a company to pay a KRW 1 million fine for failure to mee these requirements. This is in response to criticism during the parliamentary inspection that the clause was not being enforced. The NHIS plans to actively enforce this rule. Oh explained, "If fulfilling the agreement is difficult, you must communicate and consult with the NHIS beforehand. Some companies have already agreed to the penalty clause after prior consultation due to the circumstances of their contract manufacturers." Oh added, "Companies failing to submit (data) are supposed to pay KRW 1 million, but this hasn't been enforced. The NHIS is aware of this. Please comply with it going forward." Eliminating reporting requirements for new efficacy and effectiveness...will review streamlining the requirement to report dosage The current obligation for companies to report any additional efficacy and effectiveness secured overseas after signing a contract with the NHIS will be exempted starting in the first half of next year. Reporting obligations when adding a new dosage will also be streamlined. Instead, if the domestic introduction of the new strength is necessary, the details will be stated in the agreement. Oh said, "The agreement includes the obligation to report when an indication is added. We are reviewing the exemption of this reporting obligation starting in the first half of next year," and added, "We are also considering simplifying the reporting obligation for adding new dosage strengths. However, if domestic introduction of a strength already listed for overseas reimbursement is necessary, we plan to address this by explicitly stating the details in the agreement. We will be gathering opinions on this matter." The NHIS is also considering the partial disclosure of information regarding Risk-Sharing Agreement (RSA) drugs. They are currently conducting consultations on disclosing a list of drugs subject to the refund-type RSA. Oh said, "If a reimbursement decision application is made to HIRA, we will ensure that information about the refund rate can be provided. However, a non-disclosure agreement must be signed," and added, "We are also conducting consultations on publicly disclosing the list of drugs subject to the refund-type RSA." PVA 'one-time refunds' will be allowed temporarily next year...new guidelines for negotiaing scope expansion will be established Hae-hee Moon, Head of the Volume Management Unit at the Department of Drug Management.The NHIS will also improve its drug utilization management plan next year. The agency plans to restrict the operation of the "one-time refund" mechanism, which was implemented during the COVID-19 pandemic, and establish new guidelines for negotiating the expansion of a drug's usage scope. Hae-hee Moon, Head of the Volume Management Unit at the Department of Drug Management, said, "We plan to limit the products eligible for the one-time refund contract starting next year. We are currently discussing this with the Ministry of Health and Welfare (MOHW). We plan to gather opinions from the pharmaceutical association afterward." Moon explained, "We are also preparing guidelines due to the need for written instructions in the process of negotiating the expansion of usage scope. We plan to conduct consultations this week and will refine these guidelines next year." The NHIS is also reviewing plans for introducing a dual pricing system with the MOHW. Moon said, "There is a request from pharmaceutical companies to introduce a dual pricing system due to the U.S. MFN policy. We are reviewing system improvements and implementation plans with the MOHW." Revision of price cap adjustment guidelines...separate adjustments for emergency imported drugs Hyung-min Kim, Head of the New Drug Management Department.The guidelines for adjusting the ceiling price during drug price negotiations will also be partially revised. The consideration for the estimated claim amount, which was previously based on the 'claim volume and growth rate of the negotiated drug over the past 3 to 5 years,' will be changed to the 'claim amount generated by the negotiated drug over the past 3 to 5 years.' Furthermore, a clause will be added to ensure that drugs urgently imported through the Korea Orphan and Essential Drug Center (KODC) will have a separate price adjustment procedure. In addition, the clause specifying 'when there is a need for public healthcare' as an exception to the three-year limit on price adjustment applications after the initial adjustment will be further specified. Hyung-min Kim, Head of the New Drug Management Department, explained, "We will define the need for public healthcare as a request for cooperation from central administrative agencies, and revise the regulation to require the Pharmaceutical Benefit Evaluation Committee to notify the NHIS of a price readjustment application," and added, "A clause will be inserted requiring measures such as refunds if the contract falls short of the mandatory production volume agreement," emphasizing the ministry's commitment to stable supply.
- Policy
- MOHW and MOJ oppose legislating mandatory INN prescribing
- by Lee, Jeong-Hwan Nov 13, 2025 06:08am
- The Ministry of Health and Welfare and the Ministry of Justice have expressed reluctance toward a bill that would only partially mandate and enforce physician international non-proprietary name (INN) prescribing for government-designated drugs with unstable supply. Despite this being President Jae-Myung Lee’s presidential election pledge and national policy task to eliminate public inconvenience and confusion over recurring drug shortages, the Ministry of Health and Welfare and the Ministry of Justice have issued a ‘careful review’ opinion. They cited the ongoing conflict between medical and pharmaceutical groups and emphasized the need to gather broader public consensus from patients (medical consumers) and society before moving forward. The Ministry of Justice specifically expressed concern that mandating INN prescribing for drugs designated by the Ministry of Health and Welfare as having unstable supply, instead of brand names, could eliminate pharmacists' obligation to inform patients—unlike in cases of substitution dispensing—thereby restricting patients' right to know and potentially posing significant risks to public health. It also added that the post-prescription accountability remains unclear. This conclusion stems from reviewing the opinions submitted on the 11th by the Ministry of Health and Welfare and the Ministry of Justice regarding the bill mandating INN prescribing for drugs with unstable supply (partial amendments to the Medical Service Act and the Pharmaceutical Affairs Act), proposed by Representative Jong-tae Jang of the Democratic Party of Korea. Rep. Jang's bill mandates that when prescribing drugs designated as having unstable supply under the Pharmaceutical Affairs Act, the active ingredient name must be written instead of the brand name, as an exception to the current law requiring the brand name on prescriptions. The bill also includes penalty provisions stipulating that violating the active ingredient name prescription requirement for supply-unstable drugs is punishable by up to one year of imprisonment or a KRW 10 million fine for noncompliance. Ministry of Health and Welfare·Ministry of Justice “Careful Review” Both the Ministry of Health and Welfare and the Ministry of Justice, the main ministries responsible for the bill, submitted opinions calling for careful review. Their view is that caution must be exercised in legislation, separate from the fact that it is a presidential election pledge and a national policy task of the Lee administration. The Ministry of Health and Welfare expressed sympathy with the intent of introducing INN prescribing, which aims to ensure continuity of patient treatment during drug supply disruptions like shortages. However, it stated that disagreements between doctors' and pharmacists' professional organizations regarding the safety and efficacy of INN prescribing must be taken into account. This is the fundamental response the Ministry has repeatedly given in past statements regarding INN prescribing. The Ministry further stated that criteria for defining drug supply instability, measures to ensure safety and efficacy of INN prescribing, and effective implementation methods for INN prescribing must first be reviewed. The intent is to further discuss whether to introduce mandatory INN prescribing for physicians or adopt an indirect approach, such as providing incentives to prescribers who use INN prescribing. The Ministry also raised concerns about the bill's penalty provisions. It cautioned that criminalizing doctors by imposing criminal penalties for not following INN prescribing when prescribing drugs with unstable supply is an approach that requires careful consideration. The Ministry of Justice provided more specific reasons for playing caution regarding the bill. First, it stated that designating a drug as having an unstable supply would force doctors to prescribe by INN instead of brand name, which would have the effect of broadly permitting substitution dispensing regulated by the Pharmaceutical Affairs Act. The current Pharmaceutical Affairs Act’s substitution dispensing provisions require informing the patient of the substitution and obtaining prior consent from the prescribing physician or providing post-notification within one day. The Ministry of Justice opined that under Rep Jong-tae Jang’s bill, the obligation to inform patients would disappear for designated unstable-supply drugs, and the requirement to obtain prior physician consent or provide post-notification would also vanish, potentially creating problems. The Ministry pointed out, “There would be no need to inform patients, and as notification or prior consent from physicians is not required, this may potentially be a significant risk to public health. This restricts patients' right to know and also leaves unclear whether prescribers would bear responsibility after the fact.” KMA·KHA “Oppose”…KPA “Favor” The Korean Medical Association (KMA) and the Korean Hospital Association (KHA) voiced strong opposition. The KMA argued that mandating INN prescriptions is excessive legislation, as it cannot be a fundamental solution to unstable drug supply issues, yet it stipulates penalties of up to 1 year in prison or fines of up to KRW 10 million for non-compliance. It further argued that forcing doctors to INN prescribe drugs with unstable supply without considering patient conditions disregards public health rights. The KMA also contended that mandating INN prescribing undermines patient safety and treatment continuity, completely infringes on physicians' prescribing rights, and violates the fundamental principle of separating medical and pharmaceutical practices. The KMA stated, “A physician's diagnosis and prescription constitute a professional medical act that comprehensively considers individual patient characteristics, including disease status, underlying conditions, presence of drug allergies, past drug reactions, and ease of administration. When prescribing drugs, physicians select specific products—including the most suitable dosage form, strength, excipients, and coating technology—based on the specific disease and patient characteristics. Forcing INN prescribing hinders patient treatment, infringes on physicians' prescribing rights, and undermines the principle of separation of medical and pharmaceutical services." The KHA also opposed the measure, stating that instability in drug supply arises from multiple causes, such as raw material shortages, production plant issues, distribution problems, pricing, and increased demand due to specific disease outbreaks. KHA argued that legally mandating INN prescribing would be insufficient to resolve the problem and could potentially infringe on physicians' prescribing rights. The KHA emphasized that establishing comprehensive national policies would be necessary to create an environment enabling a stable drug supply. It further pointed out that penalizing non-compliance with INN prescribing, even when physicians may unknowingly prescribe by brand name due to difficulties in quickly and accurately assessing drug supply situations, violates the principle of proportionality. The KHA stated, “Long-term, fundamental measures must be established, such as creating a management system to prevent supply instability in advance. The penalties are excessive sanctions compared to other violations subject to the same penalties under current law, relative to the risk and illegality of the act.” The Korean Pharmaceutical Association (KPA) countered that INN prescribing is the most effective solution to respond swiftly to drug supply instability issues. The association's rationale for supporting the bill is that this legislation will reduce the social and economic waste caused by drug shortages and enable timely dispensing and medication services for patients. Specifically, the association argued that it is irrational for pharmacies to wait solely for a specific brand (product) to arrive when equivalent generic alternatives are available. It also expects the bill to alleviate the burden on pharmacies, which must stock medications from multiple pharmaceutical companies to accommodate individual prescriptions from different medical institutions, even for the same active ingredient. The KPA stated that Representative Jang’s bill does not contradict the existing legal framework, as the Medical Service Act and its enforcement regulations already permit INN prescribing. It also argued that distrusting or claiming differing efficacy for generics approved and registered by the Ministry of Food and Drug Safety (MFDS) is unscientific. The KPA emphasized, “When an INN prescription is issued, even if the supply of a specific pharmaceutical company's drug is unstable, the same active ingredient medication needed by the patient can be dispensed on time, preventing treatment gaps. This allows patients to obtain their prescribed medication anywhere, strengthening public access to medicines and their right of choice.” Welfare Committee expert committee presents divergent views on generic efficacy equivalence The National Assembly Health and Welfare Committee’s expert committee acknowledged that legislation has a valid aspect, as mandatory INN prescribing could help stabilize drug supply. However, it also presented points to consider during legislation. Unlike substitution dispensing, INN prescribing eliminates the need for prior physician consent or post-dispensing notification by pharmacists. In this sense, the expert committee noted that the differing opinions surrounding the therapeutic equivalence of identical-ingredient drugs (generics) must be examined. The expert committee stated, “Those affirming the therapeutic equivalence of identical-ingredient drugs maintain that generics undergo the Ministry of Food and Drug Safety's strict approval and review system, thereby ensuring recognized bioequivalence. “Conversely, those who deny equivalence argue that even with the same active ingredient, differences exist between products in formulation, dosage, excipients, coating technology, etc. They contend that when patients take a different product than before, it increases the potential for drug side effects and may lead to uncertainty in treatment efficacy.” The expert committee also pointed out that the Ministry of Health and Welfare recently clarified the requirement for post-substitution notification. It noted that the revised Pharmaceutical Affairs Act, which aims to enhance information sharing between doctors and pharmacists, passed the National Assembly, was submitted to the government, and was promulgated. Finally, it stated that the penalty clause, which imposes imprisonment for up to one year or a fine of up to KRW 10 million for violations, should also be examined for potential excessiveness. The expert committee stated, “Recently, concerns have been raised that imposing excessive penalties for administrative violations burdens criminal justice agencies and creates a large number of citizens with criminal records. Consequently, legislative reforms are underway to convert fines for minor administrative law violations into administrative penalties. In the exceptional situation where INN prescribing is mandated for unstable supply drugs, it is necessary to examine whether imposing criminal penalties for prescribing by brand name due to lack of awareness constitutes excessive punishment for violating administrative order.”
- Policy
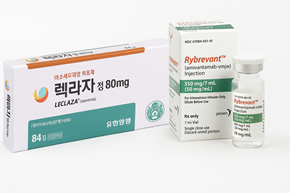
- Yuhan–Janssen join forces to tackle lung cancer
- by Jung, Heung-Jun Nov 12, 2025 06:19am
- As Yuhan Corporation and Janssen Korea join forces to co-promote their lung cancer treatments, securing reimbursement for the combination therapy has emerged as the next major hurdle to fully realize their market synergy. Janssen Korea’s non-small-cell lung cancer drug Rybrevant (amivantamab) was granted reimbursement as monotherapy by the Cancer Disease Deliberation Committee (CDDC) in September, but its combination with carboplatin or lazertinib did not receive reimbursement approval. According to industry sources on the 10th, Janssen Korea has submitted a request for reconsideration to the CDDC regarding the unestablished reimbursement criteria. At that meeting, Rybrevant’s reimbursement was not established for use in ▲combination with carboplatin and pemetrexed as first-line therapy for adults with locally advanced or metastatic EGFR exon 20 insertion-positive NSCLC, and in ▲combination with lazertinib for adults with EGFR exon 19 deletion or exon 21 (L858R) substitution mutation-positive NSCLC. However, Janssen Korea applied for a reimbursement re-evaluation for the combination therapy with carboplatin and pemetrexed 3 weeks after the CDDC decision. A cost-effectiveness review is underway for the re-evaluation. Also, with its combined use with Leclaza, which is also accumulating clinical evidence, the company’s attempts for reimbursement listing are expected to continue. Recently, the National Comprehensive Cancer Network (NCCN) also prioritized the combination therapy of Leclaza + Rybrevant as a first-line treatment for EGFR-positive non-small cell lung cancer. Given Rybrevant’s high price, public demand for its reimbursement had been strong, with a national petition for reimbursement gathering over 50,000 signatures, leading to formal discussion in the National Assembly earlier this year. During the latest NA audit, multiple lawmakers again raised questions about its reimbursement timeline. Rep. Nam-Hee Kim (Democratic Party of Korea) specifically urged the Ministry of Health and Welfare (MOHW) to establish reimbursement criteria for first-line treatment and expedite insurance coverage. In response, the Ministry stated, “The pharmaceutical company has applied for a re-evaluation of reimbursement criteria for the first-line treatment indication that had not been set the last time. We plan to proceed with the process and determine reimbursement eligibility.” Meanwhile, Janssen Korea, the domestic pharmaceutical subsidiary of Johnson & Johnson, announced on the 10th a joint promotion plan with Yuhan Corporation for the combination therapy of ‘Leclaza (lazertinib)’ + ‘Rybrevant (amivantamab)’.
- Policy
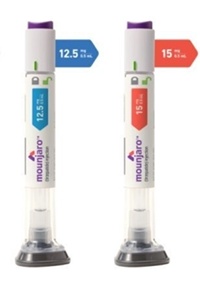
- NA requests rapid reimb listing for diabetes drug Mounjaro
- by Jung, Heung-Jun Nov 11, 2025 06:08am
- The Ministry of Health and Welfare (MOHW) announced that it will reflect the results of the October expert advisory meeting in reimbursement criteria for Mounjaro (tirzepatide), Eli Lilly’s dual GIP/GLP-1 receptor agonist, following growing calls for its rapid inclusion under the National Health Insurance for type 2 diabetes. The ministry also stated it is working to ensure the smooth reimbursement process for Ozempic (semaglutide), Novo Nordisk’s GLP-1 receptor agonist, which was reviewed by the Drug Reimbursement Evaluation Committee in October. On the 10th, Miae Kim, a lawmaker from the People Power Party, inquired about the current status of the reimbursement application process for Mounjaro for type 2 diabetes during a written inquiry session for the Ministry of Health and Welfare's comprehensive audit. She also questioned the plans for utilizing the expedited listing system, which reflects international drug prices and reimbursement cases. According to the Ministry of Health and Welfare, Mounjaro applied for reimbursement approval in March last year and was discussed by the Drug Reimbursement Criteria Subcommittee in May of the same year. Subsequent evaluations on its reimbursement adequacy were conducted in August and December 2023, and March and June 2024 by the Cost-Effectiveness Evaluation Subcommittee. The Ministry explained, “The company applied for reimbursement of Mounjaro in March last year and is currently undergoing a review on its reimbursement adequacy. The drug is being reviewed in accordance with HIRA regulations, referencing factors such as its listing status in other countries, listed prices, and coverage criteria.” It added, “To ensure appropriate use of the GLP-1 agonists Mounjaro and Ozempic for diabetes patients, HIRA held an expert advisory meeting last October to discuss patient selection criteria and evaluation methods. The findings will be reflected in the drug's reimbursement criteria.” Rep. Seo Mi-hwa of the Democratic Party of Korea also inquired about the Ministry's stance on reviewing insurance coverage for new drugs to strengthen diabetes management. The Ministry responded, “We agree that the health insurance coverage of new diabetes drugs should be actively reviewed. Currently, GLP-1 receptor agonists Ozempic and Mounjaro are undergoing the listing process. We will closely monitor to ensure the listing process proceeds smoothly and continue our efforts to improve treatment accessibility.”
- Policy
- Cancer & orphan drugs apply for reimb within 1 week of apv
- by Jung, Heung-Jun Nov 10, 2025 06:10am
- Anticancer drugs and rare disease drugs that were approved in Korea this year are racing to apply for reimbursement as quickly as within a week. Six rare/serious disease treatments approved by the Ministry of Food and Drug Safety this year are undergoing reimbursement review, excluding those subject to the approval-evaluation-negotiation system. According to industry sources on the 7th, multiple new drugs, including those designated under the Global Innovative product on Fast-Track (GIFT) scheme, are applying for reimbursement quickly after approval. Takeda Korea’s Fruzaqla (fruquintinib), which was approved in March for metastatic colorectal cancer, was submitted for reimbursement last month and is currently being assessed for clinical benefit. The drug, designated GIFT No. 20, was specifically requested for expedited coverage during this year’s NA MOHW audit as a third-line therapy for metastatic colorectal cancer. Qalsody (tofersen), Biogen’s amyotrophic lateral sclerosis (ALS) treatment and GIFT No. 31, received approval on August 20 and was immediately filed for reimbursement to expand its prescription. Imdelltra (tarlatamab), Amgen Korea’s small-cell lung-cancer drug (GIFT No. 25), was approved on May 30 and submitted for reimbursement on August 28. Augtyro (repoterrectinib), Bristol Myers Squibb’s therapy for metastatic non-small-cell lung cancer and solid tumors, followed a similar path — approved in June, filed 2 months later. Gilead Sciences’ Yescarta (axicabtagene ciloleucel), a CAR-T therapy for diffuse large B-cell lymphoma, and Janssen Korea’s Opsynvi (macitentan + tadalafil) for pulmonary arterial hypertension, both received approval in July and August, respectively, and each applied for reimbursement within just 1 week of approval. Opsynvi is now undergoing a cost-effectiveness evaluation. Industry analysts attribute this accelerated pace partly to the current administration’s policy direction under President Jae-myung Lee, which emphasizes improving patient access to orphan and severe-disease treatments. However, even with early reimbursement filings, such drugs typically face lengthy review cycles as significant time is required for clinical utility and cost-effectiveness reviews. Even if they clear the threshold of proof, a protracted drug price negotiation process remains. Currently, 33 drugs that applied for reimbursement last year and this year remain under review, suggesting fierce behind-the-scenes competition among drugs for priority listing.
- Policy
- Budget cut 20% despite increased COVID-19 vaccine uptake
- by Lee, Jeong-Hwan Nov 10, 2025 06:08am
- The Korea Disease Control and Prevention Agency (KDCA) has cut the national immunization program (NIP) budget for COVID-19 vaccines for the 2026-27 season by 20% compared to the previous year, raising societal concern over potential drops in immunity among high-risk groups like the elderly, increased mortality risk, and the possibility of early vaccine stock depletion. Moreover, the KDCA’s 20% cut to the COVID-19 NIP budget was based on the 2023-2024 season's vaccination rate of 42%, failing to reflect the 48% rate from this season (2024-2025 season). This has drawn criticism as an administrative decision that undermines effective epidemic prevention. The National Assembly Budget Office pointed out that both the number of vaccinated individuals and the coverage rate are on the rise, and has urged the KDCA to raise its target vaccination rate and revise the budget accordingly. The Budget Office recommends increasing the budget from the government’s draft of KRW 175.8 billion to KRW 200.8 billion – a KRW 25 billion increase. On the 10th, the National Assembly Health and Welfare Committee will review the KDCA budget, including the COVID-19 vaccine NIP budget, during its plenary session. The COVID-19 vaccine NIP covers high-risk groups - persons aged 65 and older, residents in communal or institutional facilities aged 6 months to 64 years, and immunocompromised individuals – with the aim of securing immunity and preventing severe illness and death. The KDCA set the 2026 NIP budget based on a vaccination rate of 42%. But looking at recent seasons, the number vaccinated was 2.90 million (39.3%) in 2022-23; 4.10 million (41.3%) in 2023-24; and 4.97 million (47.8%) in 2024-25, it was for the 65+ age group, continuing an upward trend. The major issue highlighted by experts and the Budget Office was that, despite rising vaccine uptake, the KDCA’s budget estimate remains anchored to the 42% figure from the 2023-24 season. Given statistics showing the 2024-2025 season vaccination rate approaching 48%, experts and the Office believe that setting the budget based on an incorrect baseline will inevitably lead to early vaccine depletion if the vaccination levels stay the same or increase, making it impossible to achieve the target vaccination rate. The argument is that recognizing the vaccine budget as an investment rather than an expense and rationally increasing the budget for infectious disease response is necessary to prevent potential outbreaks among high-risk groups and increases in mortality rates. In fact, 5.3 million doses of COVID-19 vaccines were secured this year based on last year's vaccination rate. However, increased uptake due to simultaneous COVID-19 and flu vaccination campaigns by health authorities and local governments led to vaccine shortages at some medical institutions just 2 weeks after the National Immunization Program (NIP) began. The NIP is a public health investment to protect citizens' lives. Prioritizing long-term effects over short-term costs will lead to socioeconomic benefits, including reduced healthcare expenses, alleviated burden on the medical system, and maintained national productivity. Furthermore, since the 2023 declaration of endemic status, COVID-19 has continued to mutate repeatedly, with the number of hospitalized patients and mortality rates constantly being reported. According to the KDCA’s infectious disease portal sample surveillance results, the number of COVID-19 hospitalizations last year was approximately 3 times that of influenza hospitalizations. The mortality rate was also higher for COVID-19 hospitalized patients at 5.97%, compared to 3.75% for influenza hospitalized patients. In the National Assembly, lawmakers, including Representative Jin-sook Jeon and Representative Nam-hee Kim of the Democratic Party of Korea, have been scrutinizing the KDCA's unreasonable NIP budget cuts. In response, the NA Budget and Planning Office has proposed the need to increase next year's NIP budget to reflect the rising trend in COVID-19 vaccination numbers and rates. The request is to increase the budget by KRW 25 billion, raising it from the KDCA's budget of KRW 175.8 billion (based on a 42% target vaccination rate) to KRW 200.8 billion (based on a 48% vaccination rate). The Budget and Planning Office analyzed, “Considering the recent trend of increasing COVID-19 NIP vaccinated individuals and vaccination rates across all 3 seasons, there seems to be a need to increase the project budget. Calculating the budget based on raising the target vaccination rate from 42% to 48% (a 6 percentage point increase) results in an estimated KRW 200.8 billion, an additional KRW 25 billion .”
- Policy
- ‘No drugs are benefitting from Korea's dual pricing scheme'
- by Jung, Heung-Jun Nov 10, 2025 06:08am
- Although a separate contract system was introduced for dual pricing of drugs in March this year, following a Ministry of Health and Welfare notice, no drugs are currently subject to the dual drug pricing system, prompting the government to review expansion plans. The NHIS stated it is reviewing with the government on expanding the scope of drugs eligible for the separate contract system. On the 7th, Democratic Party of Korea lawmakers Young-seok Seo and Byeong-hoon So submitted written inquiries during the NHIS’s comprehensive NA audit, seeking clarification on the direction and intent behind expanding the dual pricing policy. The Ministry of Health and Welfare had enacted a new notice titled “Evaluation criteria for drugs requiring assessment based on their impact on healthcare” in March, thereby introducing the separate contract system. Rep Young-seok Seo questioned the drugs currently subject to the system and the operational plan. The NHIS responded, “To be subject to the separate contract system, a drug must satisfy all 3 conditions: being an innovative new drug developed by an innovative pharmaceutical company, being approved under the expedited review system, and having undergone domestic clinical trials. Currently, there are no drugs that meet all the criteria. It added, “We are reviewing with the government and other stakeholders the expansion of drugs subject to the separate contract system to facilitate the early introduction of innovative new drugs for patient treatment access.” Representative Byeong-hoon So inquired about plans for expanding the dual pricing system. He also questioned whether plans exist to ensure fiscal stability when expanding the refund-type contract system. The NHIS stated, “Considering the enhancement of health insurance sustainability and the promotion of an innovative industrial ecosystem, we will consult with the government to improve the drug listing system, including expanding the separate contract system, and establish a balanced roadmap.”
- Policy
- The application for selective reimb of new drugs is stalled
- by Jung, Heung-Jun Nov 07, 2025 06:17am
- Roche Korea's 'Perjeta (pertuzumab),' a breast cancer treatment, was not on the recent Cancer Disease Review Committee (CDRC) agenda, despite expectations, raising questions about the background of its exclusion. One analysis suggests that the government's policy shift toward strengthening access to rare and severe disease treatments may have contributed. The view is that the Health Insurance Review & Assessment Service (HIRA) must exercise caution in applying selective reimbursement to drugs, as it aligns with impending changes to government policy. According to industry sources on the 5th, discussions on establishing reimbursement criteria for Roche Korea's HER2-positive breast cancer treatment, Perjeta, were not considered in the recent CDRC meeting, reportedly due to an application for selective reimbursement. The CDRC meeting results determined the reimbursement status for 6 products from 5 companies. Perjeta, which was expected to be on the agenda, was not included in the list of drugs discussed. Perjeta is currently applicable for selective reimbursement (with a 30% patient co-payment) for neoadjuvant (pre-operative) therapy for early breast cancer. It is reported that the company has now also applied for selective reimbursement for the post-operative therapy indication. Selective reimbursement was first implemented in 2018 under the previous Moon Jae-in administration as part of its healthcare policy to provide coverage for non-reimbursed treatments. At the time, the government planned a 3-year timeline for anti-cancer drugs and a 5-year timeline for general drugs under the Moon Jae-in Care policy, aiming to reduce patient burden for drugs with high social demand but difficult full reimbursement. With this policy, some new drugs, including anti-cancer agents, received selective reimbursement, but attempts to enter this category have been rare since. Most recent changes involve adjustments to the patient co-payment ratio for drugs already under selective reimbursement. Looking at the current list of reimbursed drugs, 123 items are subject to selective reimbursement. Excluding 97 choline alfoscerate products, which were transitioned to selective reimbursement (with an 80% co-payment) last September, the number of new drugs granted selective reimbursement is small. No new drugs have been granted selective reimbursement this year. The Lee Jae Myung administration is currently pushing a plan to expand access to treatment for rare and severe diseases. Although specific implementation plans have not been formalized, a systemic reform is anticipated. Consequently, analysis suggests that the implementation of selective reimbursement for new drugs, which was part of the previous administration's 3- to 5-year plan, is being delayed to align with the latest, changing policy direction.
- Policy
- MFDS to ease orphan drug designation eligibility criteria
- by Lee, Tak-Sun Nov 06, 2025 06:32am
- Yu-Kyoung Oh, Minister of the MFDS, is speaking at the As the criteria for Orphan Drug Designation are set to be significantly eased, this is expected to expand the introduction of rare disease treatments currently not approved in Korea. The Ministry of Food and Drug Safety (MFDS) plans to revise the regulations by February of next year. The MFDS announced this during the 'Top 50 Food and Drug Safety Tasks National Briefing Session' held on the 5th at the International Conference Hall on the first floor of the Seoul Foundation of Women and Family in Dongjak-gu, Seoul. The relaxation of the Orphan Drug Designation requirements was introduced as the very first of the seven key projects. Yu-Kyoung Oh, Minister of the MFDS, said, "We will ease the designation requirements for rare drugs not yet approved in Korea to expand treatment opportunities for patients." Currently, a drug is designated as an orphan drug if the prevalent patient population is 20,000 or fewer and if comparative data are available with an alternative medicine. During this process, it is required to submit data demonstrating that the drug is significantly safer and more effective than existing alternatives, but the industry reports difficulty providing this documentation. There have been calls to ease designation requirements to expand treatment options for rare disease patients with limited or difficult treatment options. In response, the MFDS plans to revise the criteria. Under the revised plan, a drug will be designated as an orphan drug simply by being used for the treatment or diagnosis of a rare disease, effectively eliminating the requirement to submit comparative data against an alternative therapy. The exemption of the superiority requirement for orphan drug designation is already unnecessary in countries like the United States, Switzerland, and Taiwan. The relaxation of the orphan drug designation will be implemented through the revision of the 'Regulations on Orphan Drug Designation' planned for February of next year. In addition, the MFDS plans to tag medications currently imported directly by patients for self-treatment as urgently imported drugs to ensure proper inventory stockpiling and supply. The policy aims to sequentially transition over 10 items annually, starting next year. To achieve this, the MFDS plans to secure the necessary budget through inter-ministerial consultation and expand the scope of the related program. The seven key tasks also include: ▲Operating a hotline for rapid and convenient one-stop preliminary consulting ▲Faster announcement of harmful food information via 'consumer-customized SNS' ▲Expanding treatment opportunities for innovative anti-cancer drugs by improving clinical trial participant requirements ▲Quick confirmation of safe information on dietary supplements via QR code ▲Rapid safety management of contaminants in livestock products (meat) using AI ▲Establishing clear standards for safe consumption of decaffeinated coffee. The task of improving anti-cancer clinical trial participant requirements involves establishing review criteria for early-stage anti-cancer clinical trials. This is intended to expand patient choice, invigorate anti-cancer clinical trials, and support the development of treatments for intractable cancers in situations where a cure is difficult. The MFDS plans to issue the 'Considerations for Participant Selection in Early-Stage Anti-Cancer Clinical Trials' guideline in December, which will clearly present the review criteria for clinical trials involving patients with established alternative treatments. Minister Oh emphasized that through these seven key tasks, the MFDS aims to "add security to food and drug safety so that the daily lives of the people can be healthier."
- Policy
- 'Rebate points deduction system' has been stalled
- by Lee, Jeong-Hwan Nov 04, 2025 06:13am
- The revision of South Korea's Innovative Pharmaceutical Company Certification System, which the Ministry of Health and Welfare (MOHW) is pursuing to foster the Korean pharmaceutical industry, is stalling. This is likely due to arguments for and against 'conversion to a rebate scoring system.' Following agreement with the pharmaceutical industry, the MOHW had tentatively finalized a revision to switch the 'one-strike-out' rule for innovative pharmaceutical companies caught giving illegal pharmaceutical rebates to a 'points deduction system.' However, due to recent concerns about revising the scoring system, the MOHW has yet to decide. The MOHW is criticized by opponents who argue that the current one-strike-out rule is a "poison clause" and an excessive·dual regulatory burden for companies penalized for past rebate incidents. In contrast, the counter-argument is that it is illogical to grant the Innovative Pharmaceutical Company designation, along with tax and drug price benefits, to unethical and unlawful pharmaceutical companies. Some in the pharmaceutical industry even express concern that the conversion of the rebate penalty rule from a penalty to a points system, which had been discussed positively by the Yoon Suk Yeol administration until the inauguration of the Lee Jae Myung administration, might not be pursued. The MOHW is internally reviewing the final proposal and the timing for the administrative pre-announcement of the certification system on November 2. MOHW delays the administrative pre-announcement for revision from October The MOHW had planned to complete the administrative pre-announcement procedure for the certification system revision last month (October), aiming for implementation in January of next year. Specifically, this involved amending the 'Regulations on the Certification of Innovative Pharmaceutical Companies (Notice)'. However, the ministry failed to proceed with the notice revision because it could not reach an internal conclusion on provisions such as converting the penalty for revision-violating pharmaceutical companies to a points deduction system. Consequently, the administrative pre-announcement of the revision was delayed, making the planned January implementation of the revision impossible. Pharmaceutical companies caught giving rebates, dispute over certification The point of the argument is a regulatory proposal that would ease penalties for innovative pharmaceutical companies that previously provided illegal drug rebates. This involves differentiating and converting the current standard, which immediately revokes the designation, to a lesser penalty, such as a '10-point deduction'. Pharmaceutical companies have long maintained that the Innovative Pharmaceutical Companies system, intended to encourage domestic new drug R&D and increase the potential for new drug creation, is burdened by excessive regulation that allows the certification to be revoked instantly for a debate violation. The MOHW had tentatively decided to partially reflect the pharmaceutical industry's views by abolishing the one-strike-out rule and changing the disqualification criteria to a scoring system that would deduct points from companies' scores for rebates. A 10-point deduction during the certification review for a company found to have provided rebates was highly likely. This decision by the MOHW was made during the Yoon administration, and according to the original plan, the reform, which included converting the rebate penalty to a points system, should have been finalized and revised during the first quarter of this year. However, the situation surrounding the revision changed with the transition of government. The argument gained traction that the conversion to a points system could have a negative effect, softening vigilance against illegal drug rebates or hindering the deterrent effect against unlawful activities. Accordingly, Vice Minister of Health and Welfare Lee Hyung-hoon and others were reportedly instructed to review and reconsider the detailed proposal for the rebate points deduction system. Will the scoring system be abandoned? The conversion of the rebate penalty to a points deduction system is an administrative reform strongly requested by domestic and international industry stakeholders. With news emerging that the MOHW is struggling with the transition to the points system, some parts of the pharmaceutical industry are even speculating that the conversion of the rebate penalty might be abandoned entirely. The pharmaceutical industry is considering the possibility that the MOHW may set more specific, stringent criteria for the 10-point deduction proposal. For instance, some in the pharmaceutical industry anticipate that the current regulation will be further refined into a cumulative strike-out system, where the deduction points would be increased based on the number of violations and the amount of rebates: a 10-point deduction for the first violation, 15 points for the second, and certification revocation for the third. Another side of the pharmaceutical industry predicts that the transition to a points deduction system will be abandoned. This is due to prevailing social opposition, which argues that implementing the reform could grant re-certification opportunities to pharmaceutical companies whose certification was previously canceled due to rebates (despite the penalty period), which would be socially unacceptable. In fact, during this year's parliamentary inspection, Democratic Party Rep. Kim Yoon had planned to question the CEO of a domestic pharmaceutical company as a witness regarding the appropriateness of granting re-certification opportunities and associated benefits to companies involved in rebates, but the subpoena for the witness was later withdrawn. There is also an argument for implementing the rebate deduction system, but applying the one-strike-out rule (revocation of certification) only to pharmaceutical companies that provide rebates after the new system's introduction date. This logic attempts to reflect the claim that revoking certification based on old illegal rebate incidents is overly archaic and excessive regulation, while still securing a deterrent effect against future rebate activities. The certification system revision, which appeared to be progressing smoothly, has encountered an unexpected barrier, drawing the attention of both the pharmaceutical and healthcare sectors toward the MOHW's administration. An official from a mid-sized domestic pharmaceutical company said, "It's true that there were strong complaints that the rebate revocation rule contradicted the purpose of the Innovative Pharmaceutical Company Certification System, which is to expand benefits for R&D-focused pharmaceutical companies." He added, "I understand that the MOHW accepted this, decided to switch to a points system, and even went through the deliberation of the MOHW. However, the administrative delay, coupled with the change in administration and the appointment of new ministers, altered the atmosphere." The official added, "The MOHW is reconsidering because of the growing perception that the conversion to a rebate deduction system could be viewed externally as regulatory easing that partially condones illegal activities by pharmaceutical companies." He said, "We cannot rule out the possibility that the points system transition will be abandoned. Even if the conversion proceeds, we hear that the direction will likely be to clarify the disqualification criteria for companies whose certification has already been revoked or to increase the severity of the deduction penalties." Minister of Health and Welfare Jeong Eun Kyeong stated during this parliamentary inspection regarding the revision, "The MOHW is internally reviewing the comprehensive drug pricing system revision proposal as well as the Innovative Pharmaceutical Company Certification System improvement plan."