- LOGIN
- MemberShip
- 2025-12-21 16:28:49
- K-Bios busy developing new CAR-NK cell therapies
- by Son, Hyung Min | translator Alice Kang | 2025-03-17 06:00:04
The domestic pharmaceutical industry is initiating clinical trials for cell therapies and confirming new possibilities.
GC Cell, Isu Abxis, Vaxcell Bio Therapeutics, GI Cell, and HK Inno.N are developing CAR-NK (chimeric antigen receptor) cell therapies.
CAR-NK, which is derived from allogeneic cells, has the advantage of being able to compensate for side effects caused by the use of CAR-T cell therapies.
CAR-T induces cytokines such as interleukin (IL) associated with neurotoxicity, but activated NK cells generally produce interferon-gamma (IFN-γ) and granulocyte/macrophage colony-stimulating factor (GM-CSF).
As such, CAR-NK cell therapies are less likely to cause cytokine release syndrome (CRS) and neurotoxicity.
CAR-T cell therapy is an immune cell therapy drug created by combining genetic information that expresses CAR into the patient's T cells.
CAR-T has been proven effective in hematologic cancers and has been successfully commercialized, but its disadvantages include the lack of solid tumor indications, complex production process with CRS side effects, and high cost.
GC Cell drives the development of CAR-NK cell therapy#EB According to industry sources on the 15th, GC Cell has recently started the first patient administration of GCC2005 (AB-205), a CAR-NK cell therapy candidate, in a Phase I trial.
GCC2005 is a CAR-NK cell therapy that has improved the short duration of existing NK cells and enhanced efficacy by co-expressing CAR and IL-15.
GC Cell has developed a method for culturing large quantities of highly active, high-purity NK cells from a small amount of cord blood.
The Phase I trial aims to evaluate the safety and tolerability of GCC2005 in up to approximately 48 patients with relapsed/refractory NK and T-cell malignancies and to determine the maximum tolerated dose (MTD) and recommended Phase II dose (RP2D).
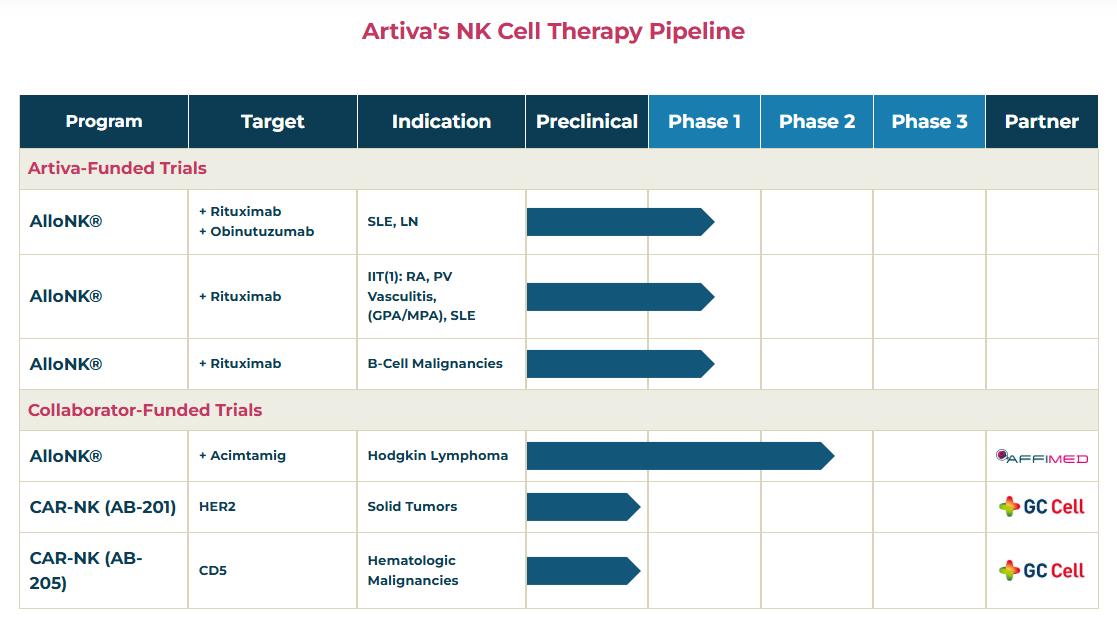
The three companies terminated their joint research agreement in June last year, but will now work together again with this agreement.
The candidate substances included in this contract utilize GC Cell’s CAR-NK platform technology and are new anticancer drug candidates that were previously developed through joint research between Artiva and MSD.
According to GC Cell, the difference between this contract and the contract terminated in June is the main entity for research and development.
CAR-NK development will be carried out by GC Cell, whereas Artiva was the main entity in the previous agreement.
GC Cell has secured global exclusive rights to the CAR-NK candidate substances and will lead the research and development (R&D) in the future.
In preclinical studies, GCC2005 showed anticancer effects in vivo in various CD5+ T-ALL models (RPMI-8402, CCRF-CEM).
GCC2005 showed higher survival rates and tumor suppression efficacy compared to the control (vehicle).
GC Cell is also developing the CAR-NK therapy AB-201.
AB-201 targets solid tumors such as HER2-overexpressing breast cancer and gastric cancer.
Artiva received approval from the US Food and Drug Administration (FDA) in 2022 for a Phase I/II clinical trial plan (IND) for AB-201.
Since then, GC Cell has been conducting clinical trials after receiving approval for the Phase 1 IND for AB-201 from the Ministry of Food and Drug Safety and the Human Research Ethics Committee (HREC) of Australia in December last year.
Through the trial, GC Cell plans to evaluate the safety and tolerability of AB-201 in patients with HER2-positive solid tumors and determine the recommended Phase II dose.
Domestic companies also confirm the possibility of developing CAR-NK cell therapies
The company's ISU104, which is under development, targets the HER3 (ErbB3) protein, which is mainly expressed in breast cancer.
Isu Abxisand Seok-Ho Kim, a professor at Dong-A University's Department of Biomedical Engineering, has developed CAR-NK cells that have been engineered to express CAR cells targeting ErbB3 from cord blood-derived NK cells.
The results of the research showed that when treating breast cancer cell lines with CAR-NK cells, the cancer cells were killed.
Furthermore, in a mouse model implanted with breast cancer cells, ISU104-CAR-NK showed the effect of reducing the size of the tumor without any particular side effects.
To date, no HER3-targeted anticancer drugs have been developed yet.
Patritumab deruxtecan, an antibody-drug conjugate (ADC) that is in Phase II clinical trials by Daiichi Sankyo and MSD, is currently developed the furthest.
Last year, Vaxcell Bio signed a business agreement with Bio Design Lab, a virus vector design company, to jointly develop CAR-NK cell therapies for the treatment of autoimmune diseases.
Previously, Vaxcell Bio has signed MOUs with Bio Design Lab, Samsung Medical Center, and others to conduct research and development of new CAR-NK cell therapies.
Under this agreement, Vaxcell Bio will provide third-generation NK cells and oversee the entire process of CAR-NK development, including research and clinical trials that meet GMP standards.
Bio Design Lab will independently design and produce lentivirus vectors, one of the core technologies in CAR-NK cell therapy development.
GI Innovation's affiliates GI Cell and Y Biologics signed a memorandum of understanding last year and began developing CAR-NK anticancer drugs.
The two companies plan to develop anticancer drugs through GI Cell's CAR-NK cell therapy development and mass culture technology and Y Biologics' antibody discovery platform technology, Nanobody.
GI Cell has completed a Phase I clinical trial in Korea for its NK cell therapy, T.O.P.
NK, for patients with solid and hematological cancers.
After proving the tolerability and safety of T.O.P.
NK, GI Cell has been conducting a Phase IIa clinical trial since last month.
GI Cell is embarking on joint research with HK Inno.N for a CAR-NK cell therapy.
HK Inno.N and GI Cell are conducting basic research on 7 targets together.
HK Inno.N is conducting two basic research projects on its own in addition to the joint research.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










