- LOGIN
- MemberShip
- 2025-12-21 16:46:23
- HLB shares plummet upon 2nd FDA rejection of rivoceranib
- by Cha, Jihyun | translator Alice Kang | 2025-03-25 05:54:19
The shares of HLB Group affiliates plummeted as HLB's new drug for liver cancer failed to enter the US market again.
The total market capitalization of HLB Group stocks evaporated by over KRW 3 trillion in a single day.
However, this did not cause a simultaneous drop in domestic bio stocks.
HLB Group's 10 listed affiliates evaporate by KRW 3 trillion in a single day upon receiving a second Complete Response Letter (CRL) According to the Korea Exchange on the 22nd, HLB closed at KRW 46,500 on the 21st.
This is a 29.97% drop from the previous trading day.
The day before, HLB's share price plunged to the price floor immediately after the opening and remained at this price until the closing time.
The market capitalization evaporated by KRW 2.6147 trillion in a single day as the stock price hit the floor.
HLB's market capitalization was KRW 8.7241 trillion based on the closing price on the 20th, but it plunged to KRW 6.1095 trillion based on the closing price on the 21st.
HLB's share price fell sharply after its new drug for liver cancer failed to enter the US market.
HLB said on its official YouTube channel at 3 a.m.
on the 21st that it had received a CRL from the US Food and Drug Administration (FDA) for its rivoceranib+camrelizumab.
In May last year, the company received a CRL completed the supplementary work, and submitted the required documents for a second review, but received another CRL.
Upon the news of HLB’s CRL receipt was announced, the share prices of HLB Group’s stocks fell across the board.
HLB Global, HLB Life Sciences, HLB Pharma, and HLB PanaGene all recorded the lowest opening price on the 21st.
The opening prices of HLB affiliates on the 21st were KRW 2,535 for HLB Global, KRW 5,990 for HLB Life Sciences, KRW 17,100 for HLB Pharma, and KRW 1,665 for HLB PanaGene, down 29% from the previous trading day.
HLB bioStep and HLB Innovation also opened at a price 26% lower than the previous day's closing price.
HLB Genex and HLB Science opened at a price 21% and 15% lower than the previous day's closing price.
The ten HLB Group’s listed affiliates showed a downward trend throughout the day.
HLB (-29.97%), HLB Life Sciences (-29.94%), and HLB Pharma (-29.92%) closed at the floor price.
HLB Global (-18.09%), HLB Genex (-15.54%), HLB Science (-14.95%), HLB bioStep (-14.71%), HLB PanaGene (-14.32%), HLB Therapeutics (-7.37%), and HLB Innovation (-6.60%), also closed at a low price.
As of the closing price on the 20th, the market capitalization of the 10 listed affiliates of HLB Group totaled KRW 12.924 trillion.
As of the closing price on the 21st, the total market capitalization of these affiliates was KRW 8.7698 trillion.
As of the closing price on the 21st, KRW 8 trillion of the market capitalization of HLB Group stocks had evaporated compared to the end of last month.
The stock prices of HLB Group's listed affiliates suddenly soared on the 27th of last month.
The analysis was that the rise was the result of the combined expectations for new drug approvals and the successive stock purchases of HLB Group Chairman Yang-gon Jin, the owner.
At the time, the total market capitalization of HLB Group stocks reached KRW 16.5843 trillion.
A similar situation occurred last year when the company received its first CRL.
The total market capitalization of the 9 HLB Group’s listed affiliates, excluding HLB Genex, which the company acquired at the end of last year, fell by KRW 5.274 trillion from the previous day on May 17 last year, when the news of the failure to obtain approval for the use of rivoceranib+camrelizumab was announced.
At an online press conference on the morning of the 21st regarding the CRL notification, Chairman Yang-gon Jin said, “I think that the shareholders as well as our employees at HLB Group are disappointed by the receipt of this CRL.
I would like to express my regrets about this and will actively communicate through meetings after the shareholders' meeting.” However, HLB’s situation did not affect the domestic bio-sector.
On the 21st, Sam Chun Dang Pharm, Kolon TissueGene, and LigaChem Biosciences showed a strong trend despite the news of the CRL for rivoceranib+camrelizumab.
On the 21st, the closing price of Sam Chun Dang Pharm was KRW 188,800, up 7.64% from the previous day.
The closing prices of Kolon TissueGene and LigaChem Biosciences on the 21st also rose 6.88% and 1.72%, respectively, from the previous day.
Peptron (+4.29%), PharmaResearch (+2.35%), and Hugel (+1.21%) also closed up.
This is in contrast to a past case in which the failure of a company to receive approval for a new drug dampened investment sentiment in the entire biotech industry.
Domestic biotech stocks fell in tandem shortly after the announcement of the suspension of the clinical trial of SillaJen's liver cancer treatment 'Pexa-Vec' in 2019 and the failure of its clinical trial on Helixmith's diabetic neuropathy (DPN) gene therapy ‘Engensis' (VM202) in 2020.
As a result, investor sentiment froze, and biotech stocks experienced dark ages for a while.
Acquired from Bukwang Pharmaceutical in 2018, final data released at ASCO last year demonstrated OS extension Rivoceranib is an oral targeted anticancer drug of the vascular endothelial growth factor receptor 2 (VEGFR2) inhibitor class, which is involved in the formation of new blood vessels in tumors.
The drug was developed in 2005 when US-based Elevar Therapeutics bought the global rights to rivoceranib from the Advenchen Laboratories.
Bukwang Pharmaceutical, which recognized the potential of rivoceranib, secured the rights to sell the drug in Korea, Europe, and Japan from Elevar Therapeutics in 2009.
HLB Life Sciences acquired the development rights to rivoceranib from Bukwang Pharmaceutical in 2018 for KRW 40 billion.
HLB then acquired the product patent rights for rivoceranib in 2020 and capitalized on it as its material.
HLB has been developing a rivoceranib+camrelizumab combination therapy with Jiangsu Hengrui Pharmaceuticals as a treatment for liver and stomach cancer.
Camrelizumab, which was developed by Jiangsu Hengrui Pharmaceuticals, is an immuno-oncology drug that inhibits the PD-1 protein expressed on the surface of immune cells (T cells), preventing them from binding to the PD-L1 receptor on the surface of cancer cells and activating immune cells.
HLB applied for an NDA to the FDA in May 2023 for the rivoceranib+camrelizumab combination as a first-line treatment for liver cancer.
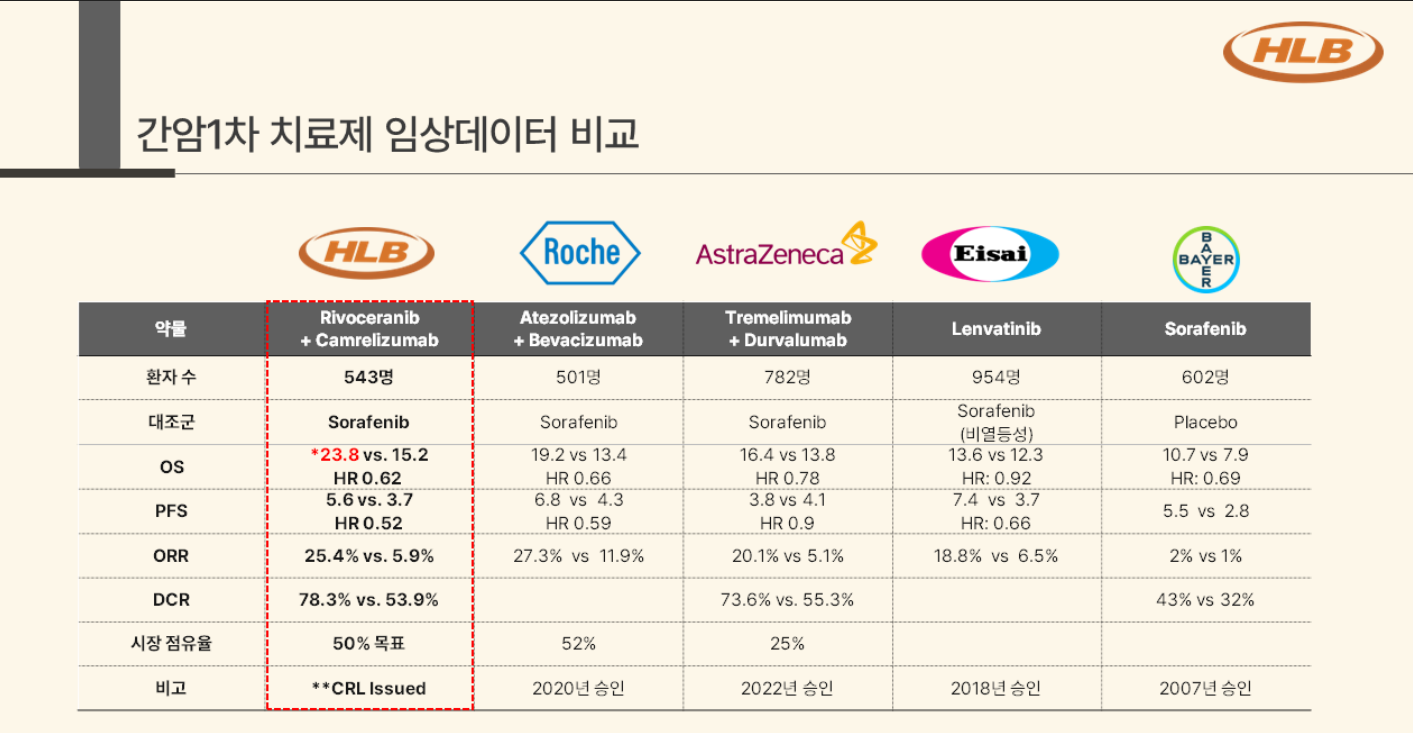
The results of the Phase III CARES-310 study, which was presented by HLB and Jiangsu Hengrui Pharmaceuticals at the 2022 European Society for Medical Oncology (ESMO) Congress, were presented as grounds for its approval.
The clinical trial was conducted to compare the efficacy and safety of lenvatinib and camrelizumab with that of Bayer's Nexavar, which is the current standard of care for liver cancer.
In the study, rivoceranib+camrelizumab recorded a median overall survival (mOS) of 22.1 months, showing improved results compared to Nexavar’s 15.4 months.
This result showed a longer OS than the 19.2 months found with Roche's combination therapy of the immuno-oncology drug Tecentriq and the targeted-oncology drug Avastin, which has been approved as a first-line treatment for liver cancer, and the OS of 16.4 months for AstraZeneca's combination therapy of the immuno-oncology drug Imfinzi and Imjudo.
The clinical trials for the combinations comparing the respective combinations with Nexavar monotherapy.
The progression-free survival (PFS) of rivoceranib+camrelizumab was 5.6 months, and the objective response rate (ORR) was 33.1%.
HLB also released final results that were even better than the clinical phase III trial results previously announced at the American Society of Clinical Oncology (ASCO) meeting last year.
According to the results announced by HLB at the ASCO Annual meeting in May last year, the OS of rivoceranib+camrelizumab was 23.8 months, which was longer than the previous results of 22.1 months.
HLB has included the additional improved data during re-submission to the FDA.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










