- LOGIN
- MemberShip
- 2025-12-21 16:46:01
- 'Onbevezy' holds the No.1 spot for the domestic mkt
- by Chon, Seung-Hyun | translator Hong, Ji Yeon | 2025-03-26 06:00:45
Samsung Bioepis' anticancer agent Onbevezy climbed to hold the No.1 spot in domestic sales as the first domestically developed biosimilar.
It has surpassed Remsima for the first time.
The quarterly sales of Onbevezy and Remsima exceeded KRW 10 billion, each competing to hold the No.1 spot.
According to the Financial Supervisory Service on March 26, Samsung Bioepis' Onbevezy generated domestic sales of KRW 45.2 billion last year, up 14.0% from the previous year.
Onbevezy chased Remsima's sales last year, KRW 44 billion, by a difference of KRW 1.2 billion, and rose to hold the No.1 spot for domestic sales of domestically developed biosimilars.

Legend: Blue-Onbevezy, Yellow-Remsima, Gray-Herzuma Onbevezy is a biosimilar to the anticancer drug Avastin.
It is an anticancer drug used to treat patients with metastatic colorectal cancer, metastatic breast cancer, non-small cell lung cancer, advanced or metastatic renal cell carcinoma, glioblastoma, epithelial ovarian cancer, carcinoma of the fallopian tube, primary peritoneal cancer, and cervical cancer.
Samsung Bioepis launched Onbevezy in September 2021 in the Avastin market.
Celltrion and Alvogen Korea also entered the market.
In 2023, Onbevezy was behind sales of Remsima by KRW 800 million, but it successfully exceeded Remsima sales last year.
Onbevezy entered the market first among other biosimilar products.
Analysis suggests that a customized marketing strategy maximized the synergy.
After obtaining the domestic approval of Onbevezy, Samsung Bioepis signed an exclusive sales agreement with Boryung, one of the Korean companies with strength in the field of anticancer drugs.
In May 2020, Boryung newly established the ONCO (anticancer) sector.
The company independently separated the sector from the prescription medicine sector.
Boryung secured sales rights to various anticancer drugs and biosimilars owned by Korean and international companies.
With the LBA (Legacy Brands Acquisition) strategy of acquiring sales rights for original anticancer drugs, Boryung secured Gemzar and Alimta.
Boryung also secured Korean sales rights for Herceptin biosimilar from Samsung Bioepis.
In Q2 2023, Onbevezy surpassed sales of KRW 10 billion, and the drug also recorded quarterly sales of over KRW 10 billion.
Remsima, which Celltrion sells, generated sales of KRW 44 billion last year, up 8.7% from the previous year.
However, it allowed Onbevezy to overtake its position.
Remsima is a biosimilar to the autoimmune disease treatment Remicade.
It was approved as the first domestically developed antibody biosimilar in 2012.
Remsima is used to treat Crohn’s disease, ankylosing spondylitis, ulcerative colitis, and rheumatoid arthritis.
Upon launch, Remsima continued to generate the highest domestic sales figure for over 10 years among domestically developed biosimilars in South Korea.
Remsima generated global sales of KRW 1.2680 trillion last year, an increase of over three-fold from KRW 420 billion in 2023.
It topped KRW 1 trillion in yearly sales, first among domestically developed pharmaceuticals.
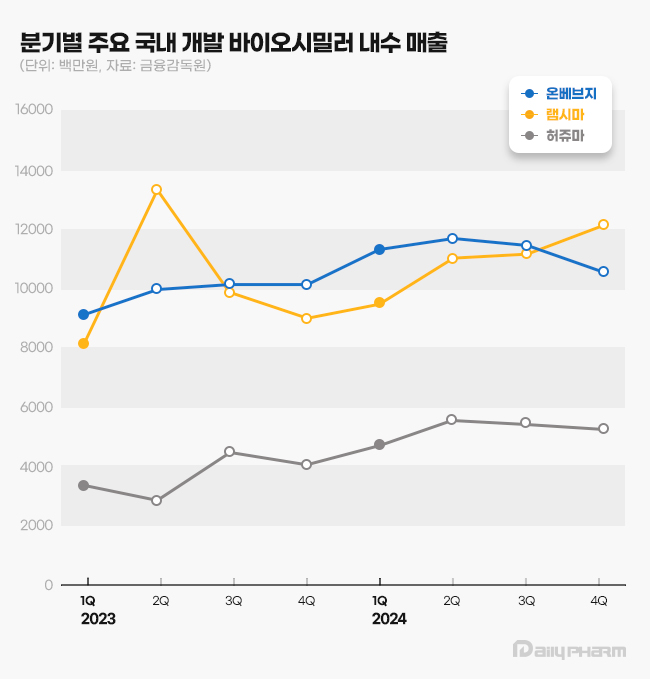
However, in Q4 of last year, Remsima reversed the trend again, indicating strong competition for the top position.
In Q4 of 2024, Remsima's sales reached KRW 12.1 billion, a 35.3% increase year-over-year (YoY), outpacing Onbevezy by KRW 1.6 billion.
In contrast, Onbevezy's Q4 sales for last year were KRW 10.6 billion, a YoY increase by 4.3%.
However, Onbevezy lost to Remsima in just six quarters.
The introduction of Korea-made biosimilars typically drives down the prices of original drugs, thereby reducing the burden on the National Health Insurance budget.
Following the listing of Onbevezy, in October 2021, the ceiling price for Avastin 0.1 g/4 mL was reduced by 30%, from KRW 330,387 to KRW 231,271, and that for Avastin 0.4 g/16 mL dropped by 30% from KRW 1,077,531 to KRW 752,746.
According to the policy, when a biosimilar enters the domestic market, the ceiling price for the original drug is lowered by 30% compared to its pre-patent-expiration level.
However, 'Products developed by innovative pharmaceutical companies·Those deemed equivalent·products developed through joint agreements between domestic and foreign pharmaceutical firms·Products for which Korea was the first approving country·Products manufactured domestically' are guaranteed to retain up to 80% of the original product's pre-patent-expiration price for both the original drug and its biosimilar.
Since Samsung Bioepis is not classified as an innovative pharmaceutical company, Avastin's price fell to about 70% of its previous level.
According to IQVIA, a pharmaceutical market research firm, Avastin recorded sales of KRW 30.9 billion in Q3 2021, but it declined sharply to KRW 22.6 billion in Q1, a drop of 27.2%.
The introduction of biosimilars, which led to a 30% reduction in the original drug's price, is analyzed to have resulted in significant savings for the National Health Insurance budget and patients' drug costs.
Among Korea-made biosimilars, Celltrion's Herzuma generated sales of KRW 21.3 billion last year, an increase of 43.4% from the previous year.
Herzuma is a biosimilar version to Herceptin.
Truxima, Celltrion's biosimilar version of MabThera, generated sales of KRW 11.4 billion last year, a drop of 23.2% from the year before.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










