- LOGIN
- MemberShip
- 2025-12-21 13:04:12
- Trials for bispecific antibodies in cancer immunotherapy
- by Son, Hyung Min | translator Hong, Ji Yeon | 2025-04-09 05:56:22
Yuhan, Hanmi Pharmaceuticals, and other domestic pharmaceutical and biotech companies have entered Phase 1 clinical trials of bispecific immuno-oncology drugs, delving into new drug development.
Bispecific antibodies are drugs that can simultaneously bind to two different antigens or two distinct antigen-binding sites on the same antigen.
Multispecific antibodies that target biomarkers simultaneously have the advantage of crossing the blood-brain barrier (BBB) by binding to receptors on its surface.
In particular, passage through the BBB is necessary for anticancer drugs to enhance drug permeability.
Recently, an increasing number of companies have been developing multispecific antibodies by combining antibodies that bind to antigens regulating immune cell activation with those that bind to tumor-specific antigens.
Yuhan·Hami enter phase 1 trials #iAccording to industry sources, on the 9th, Yuhan received IND approval from the Ministry of Food and Drug Safety (MFDS) for its bispecific antibody immuno-oncology candidate 'YH32364.' Introduced initially to Yuhan in 2018 from ABL Bio, YH32364 is now entering its first clinical study in humans.
This trial will assess the safety, tolerability, pharmacokinetics, and antitumor activity of YH32364 in patients with locally advanced or metastatic solid tumors that overexpress the epidermal growth factor receptor (EGFR).
YH32364 is designed as a bispecific antibody that simultaneously targets EGFR and 4-1BB.
EGFR is a well-known biomarker expressed in major solid tumors, including non–small cell lung cancer (NSCLC) and colorectal cancer.
By simultaneously targeting EGFR and T-cell activating 4-1BB, Yuhan aims to maximize the antitumor effect of this immuno-oncology candidate.
Hanmi Pharmaceuticals, in collaboration with Beijing Hanmi Pharmaceuticals, is currently conducting a Phase 1 clinical trial of the immuno-oncology candidate 'BH3120,' which targets both 4‑1BB and PD‑L1.
Cetuximab is an anticancer drug that targets the EGFR receptor and is used in the treatment of various cancers, including colorectal cancer, head and neck cancer, and lung cancer.
Yuhan expects YH32364, which activates immune cells through 4‑1BB action dependent on the tumor’s EGFR expression, will be effective against a broader range of EGFR‑expressing solid tumors than conventional anti‑EGFR monoclonal antibodies.

Hanmi Pharmaceuticals’ proprietary platform technology Pentambody was used in developing BH3120.
Pentambody is a next‑generation dual antibody platform technology that activates immune cells while attacking only the target cancer cells.
Because BH3120 activates 4‑1BB only in immune cells surrounding PD‑L1‑expressing tumor cells, it minimizes the toxic side effects of 4‑1BB and achieves long‑term anticancer effects that prevent recurrence.
In clinical trials, BH3120 demonstrated decoupling effects of immune activation between the tumor microenvironment and normal tissues, confirming its safety.
Hanmi Pharmaceuticals and Beijing Hanmi Pharmaceuticals also explore combining BH3120 with additional anticancer agents.
Active R&D for bispecific immune-oncology agents Korean biotech industry also focuses on bispecific antibody immune-oncology agents.
In addition to ABL Bio's YH32364 (ABL Bio candidate ABL104) transferred to Yuhan, it also has ABL501, which targets PD‑L1 and LAG‑3, and ABL503, which targets PD‑L1 and 4‑1BB.
ABL501 is a bispecific antibody designed to improve the low response rates of conventional immuno-oncology agents and treat patients with resistance by targeting two different immune checkpoint proteins, PD‑L1 and LAG‑3.
This candidate blocks the binding of LAG3-MHCII and PD‑1-PD‑L1, thereby preventing T cells from being inactivated by tumors.
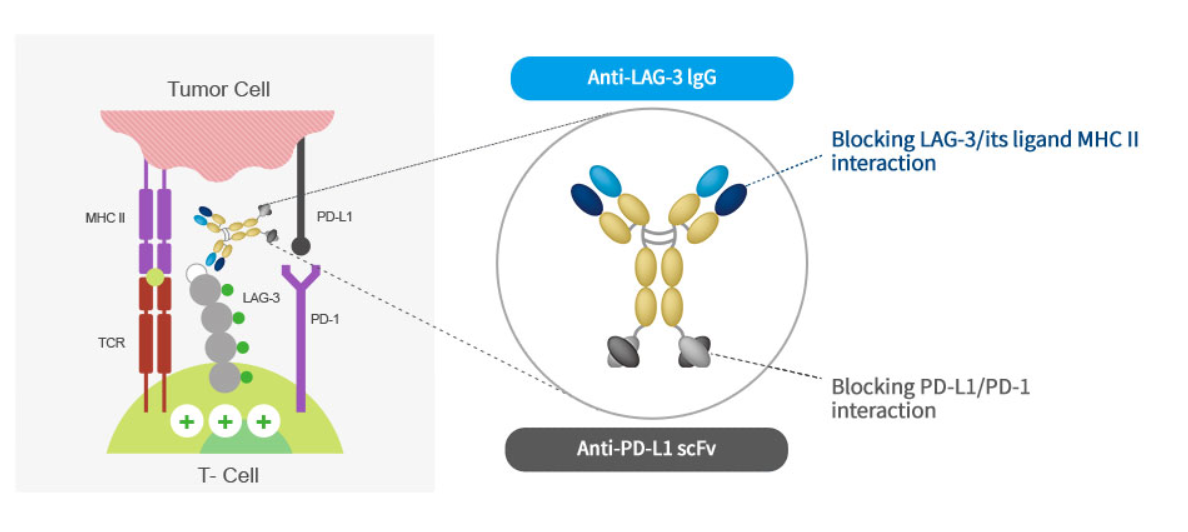
Currently, ABL501 monotherapy is being studied in a dose-escalation Phase I trial as a monotherapy.
This clinical study is funded by the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, and the Ministry of Health and Welfare (MOHW) under the National New Drug Development Support Project.
ABL Bio is also conducting a Phase 1 trial of ABL503.
Clinical data showed that the administration of ABL503 has demonstrated antitumor efficacy and confirmed its safety profile.
Notably, among the patients who achieved a complete response (CR) or partial response (PR), some had previously shown resistance to or experienced recurrence after treatment with immunotherapies targeting PD‑L1.
Among 26 patients exposed to the effective dose, responses were observed in 7 patients.
The results showed that there was one CR and six PR.
Regarding safety, after administration of ABL503, some toxicities inherent to PD‑L1 and 4‑1BB targeted anticancer agents were observed; however, these were manageable with steroid treatment or temporary treatment interruption.
Tium Bio has confirmed the efficacy of the bispecific inhibitor TU2218 in preclinical studies.
TU2218 concurrently blocks transforming growth factor (TGF‑β) pathways, which is known to interfere with immuno-oncology activity and vascular endothelial growth factor (VEGF).
The process maximizes the efficacy of immuno-oncology agents.

In a colorectal cancer model, the potential of a triple combination therapy was demonstrated.
In a colorectal cancer model, the potential of a triple combination therapy was demonstrated.
The combination of TU2218, an anti‑PD‑1 agent, and an anti‑CTLA‑4 agent exhibited superior tumor growth inhibition compared to the control group receiving a placebo plus an anti‑PD‑1 agent and an anti‑CTLA‑4 agent.
The triple combination therapy containing TU2218 achieved an 84% tumor growth inhibition rate, compared to 70% in the control group.
Additionally, TU2218, in combination with an anti‑PD‑1 agent and Renbatinib, achieved a 99% tumor growth inhibition rate.
IMC-201, developed by ImmuneOncia, is a bispecific antibody independently created using CD47 and PD‑L1.
In preclinical studies, IMC-201 strongly bound to solid and hematologic cancer cells expressing CD47/PD‑L1, while selectively acting only on the cancer cells even under conditions where red blood cells and cancer cells were co‑cultured.
Furthermore, it exhibited higher macrophage-mediated phagocytosis compared to the parental antibody, IMC-002.
In particular, in a mouse tumor model of triple‑negative breast cancer, IMC-201 demonstrated more potent tumor inhibition than the combination of the parental antibodies IMC-002 and IMC-001.
ImmuneOncia is also developing the immuno‑oncology candidate IMC-002.
IMC-002 is designed to block CD47 on cancer cells and inhibit macrophage signaling.
In a study where IMC-002 was administered to 12 patients, no drug toxicity was observed at any dose level.
Among these 12 patients, 6 achieved stable disease (SD).
The company plans to determine the recommended Phase 2 dose based on the clinical findings from the Phase 1b study.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










