- LOGIN
- MemberShip
- 2025-12-27 17:20:38
- Is MFDS going to suspend sales on nizatidine with no NDMA?
- by Chon, Seung-Hyun | translator | 2019-11-06 08:59:26
Pharmaceutical industry is walking on thin ice as the government initiated impurity investigation on stomach ulcer medicine.
Now that the U.S.
detected impurity in nizatidine, following a case in Japan, probability of finding impurity in nizatidine has gotten higher in Korea.
The industry is on high alert against the government’s possible order to suspend sales of nizatidine drug without detecting any impurity, which was the case with ranitidine.

MFDS ordered companies to submit API usage record and other archived evidences to confirm manufacturing record until Nov.
4.
The ministry seems to be investigating uses of all complete product with both Korean-made and imported nizatidine Nizatidine is an H2-receptor antagonist similar to ranitidine suspended of sales from last September.
After deciding to suspend sales of all ranitidine drugs, MFDS also set a plan to investigate similar APIs, starting with nizatidine first.
Ministry’s nizatidine usage record investigation resembles that of ranitidine’s.
On last Sept.
20, MFDS directed pharmaceutical companies to investigate ranitidine API usage record, and six days after on Sept.
26, the ministry announced sales suspension on all ranitidine items.
The industry presumes MFDS is about to announce nizatidine investigation result based on the precedent case.
And now, the industry is nervously waiting for the ministry’s decision on nizatidine items.
.Possibility of finding N-Nitrosodimethylamine (NDMA) has been raised at home and aboard, already
.A private U.S.-based research institute, Valisure unveiled their testing report on nizatidine last September and stated they have detected NDMA
.Previously, Valisure proposed regulators to recall ranitidine as it detected excessive level of NDMA in the API
.Their latest report state researchers found one-seventieth of NDMA in ranitidine was detected in nizatidine
.Japanese Ministry of Health, Labour and Welfare announced Japan-based Ohara Pharmaceutical tested their nizatidine product and detected NDMA exceeding the accepted level
.The ministry reported the company decided to voluntarily recall their products due to the issue
.On Nov
.4, the U.S
.Food and Drug Administration (FDA) released a statement about their investigation on NDMA found in ranitidine, and stated four nizatidine items from two companies had NDMA
.However, the said nizatidine drugs had NDMA within the accepted level and were not included in the voluntary recall subject group
.Some of pharmaceutical companies in Korea are promoting that their nizatidine drugs are NDMA free
.However, some have raised concern about possibility of detecting NDMA in nizatidine ingredient used in Korea
.At the moment, total nine API manufacturing plants have been registered to produce nizatidine
.Korean Medical Association (KMA) has already advised doctors to refrain from prescribing nizatidine
.In last month, KMA official said “The recent ranitidine incident has created a social turmoil, so the organization advised members to refrain from prescribing nizatidine containing drugs until MFSD announces final investigation result and its further action”
.Pharmaceutical companies are keeping a close eye on MFDS’ further action for when finding minuscule amount of NDMA in nizatidine
.The companies are afraid of the ministry ordering sales suspension on all nizatidine drugs for questionable cases of NDMA not detected from most of the complete product, and a single manufacturing unit containing minuscule amount of NDMA exceeding acceptable level
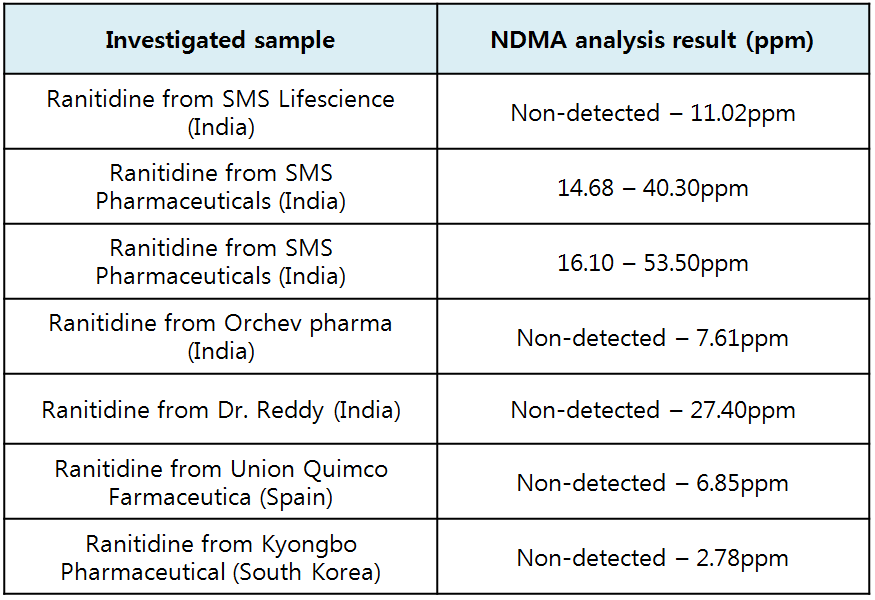
.As for ranitidine drugs, all seven manufacturing plants had NDMA surpassing acceptable level in the API, but each item manufactured in a same plant had different levels of NDMA
.For instance, some ranitidine drugs manufactured from one plant were found with unacceptable level of impurity and others were not
.
.After making the statement, the ministry ordered sales suspension on all ranitidine drugs, judging that ranitidine itself is too unstable to be consumed
.A pharmaceutical research institute, UBIST reported last year’s volume of nizatidine drug prescription for outpatient reached 25.9 billion won
.Although it would be about one tenth of the ranitidine market, some pharmaceutical companies are faced with serious damage from sales suspension on popular nizatidine products
.Pharmaceutical companies continue to urge the Korean regulators to suspend sales limited to items exceeding acceptable NDMA level like the case in the U.S
.Some of the industry is also questioning credibility of the NDMA testing methodology as same API has been detected with different levels of NDMA
.A pharmaceutical industry insider stressed, “MFDS has decided to suspend sales on valsartan and ranitidine drugs, regardless of each sample showing different level of the carcinogen
.The regulators should use more precise investigative methodology and limit penalty to items with exceeding level of NDMA only”
.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









