- LOGIN
- MemberShip
- 2025-12-27 17:20:52
- MA experts admit headquarters’ “Korea Passing” orders
- by Kim, Jin-Gu | translator Byun Kyung A | 2019-11-28 06:14:03
The government and pharmaceutical industry are showing polarizing views on the current situation and the cause of Korea Passing phenomenon.
As if they are speaking in different languages, their prospective and proposed solutions are like parallel lines never crossing each other’s path.
So what does pharmaceutical industry fear about in those two words of ‘Korea Passing’?
Daily Pharm conducted a survey on 21 Market Access experts from respective affiliation to get a better look into what the industry is trying to convey.
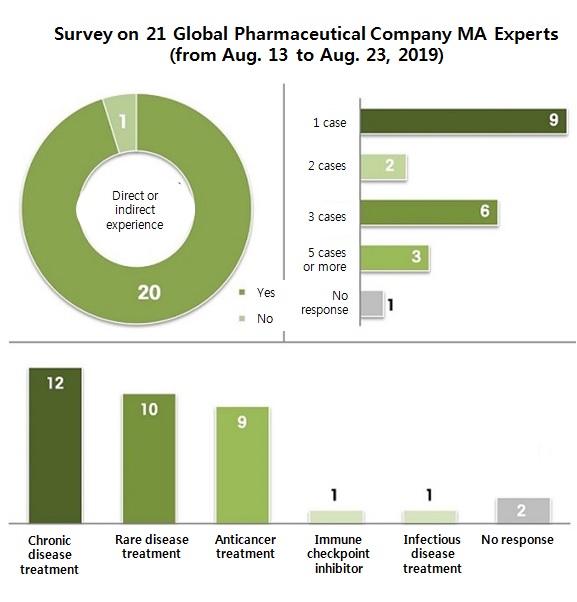
Korea Passing is not a myth, but a reality in global pharmaceutical companies First, the survey questionnaire asked if the experts have experienced Korea Passing either directly or indirectly.
Except one response, 20 said they have experienced directly or have heard of it.
For those have either experienced or heard of it, the survey questioned how many cases of Korea Passing they have come across with.
Nine said one case, and other nine said two to three cases.
Three answered five or more cases.
The average was about 4.2 cases.
Sorting by treatment type, chronic disease treatments faced Korea Passing the most with 12 cases (multiple responses allowed).
Rare disease and anticancer treatments followed with ten and nine cases, respectively.
Immune checkpoint inhibitor and infectious disease treatment each experienced once.
So far, allergic asthma treatment Xolair, antidiabetic treatment Victoza, amyotrophic lateral sclerosis (ASL) treatment Radicut, and immunotherapy Obdivo have been mentioned the most when talking of Korea Passing.
But the survey revealed more cases.
It hinted that more Korea Passing phenomena are happening behind the scenes.
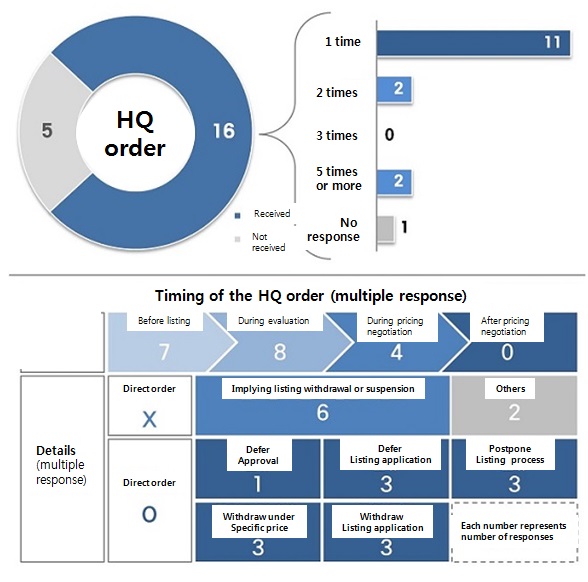
Excluding indirect experience of the phenomenon from word of mouth, the questionnaire asked if they have received an order from global headquarters to drop application or negotiation with government body.
16 out of 21, or about 76 percent of the survey participants answered they have.
11 of them said there was one such case, and four said two or more cases.
One did not answer.
Apparently, the order was given for seven items before they applied for insurance reimbursement listing, eight during reimbursement feasibility evaluation, and four during drug pricing negotiation.
On the details of the ‘order’ regarding Korea Passing, 13 answered the headquarters specifically mentioned the word “withdrawal” or “defer” during a meeting.
One said their office was ordered to defer the Ministry of Food and Drug Safety (MFDS) approval application submission.
Three items received an order to suspend reimbursement listing application submission, and other three got an order to postpone processing after applying for reimbursement listing Three answered they were given an order to pull out from negotiation when negotiating pricing went under certain price.
Moreover, three said their headquarters ordered them to unilaterally withdraw from reimbursement listing.
Not a specific order, but even six of the participants said they had to read between the headquarters’ message implying withdrawal or suspension during meetings.
Taken at face value, there have been at least 16 cases where a global headquarters has given a direct order to either withdraw or suspend government processing.
Multiple responses aside, the survey confirmed many of global headquarters are sensitive about pricing in Korea and also go as far as to directly give an order.
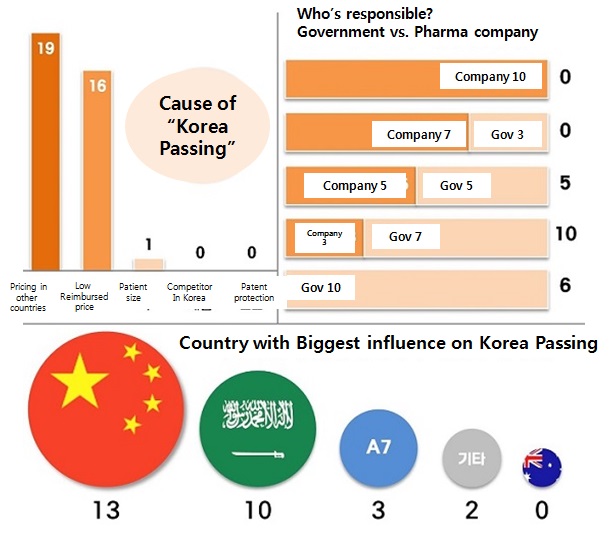
Industry admits some of their contribution The participants suspected two major reasons were behind the Korea Passing phenomenon.
19 said drug pricing in different countries, and 16 said low reimbursed price in Korea, including multiple responses.
Questionnaire asked once again to see if Korean government or pharmaceutical company played any part.
None of them said pharmaceutical companies were either entirely or mostly responsible.
However, six of them said it was entirely because of the Korean government.
Majority of the participants, or ten of them said the Korean government is mostly responsible while pharmaceutical companies were also partially responsible.
Five answered the companies and government were responsible 50/50.
Majority of the participants, or 13 of them answered China played the biggest role in Korea Passing, as expected.
Other regions like the Middle East, A7 countries and others were mentioned by ten, three and two participants, respectively.
None of them said Australia.
The Chinese government has been completely reforming its pharmaceutical insurance benefit policy for last two years.
In the process, the government has decided to include Korea as one of their international reference pricing countries.
Previously, the country has been referencing drug prices in Korea unofficially.
But when China made it official was the tipping point for the global headquarters to make more drastic decisions.
The point in time matches with right around when Korea Passing has become a social issue.
Korean pharmaceutical industry is concerned of multinational drug maker headquarters making decisions to postpone or withdraw reimbursement listing application due to Chinese market.
Besides China, the Middle East was also played a crucial role in Korea Passing.
Among the Middle Eastern countries, Saudi Arabia is reportedly referencing Korean drug pricing.
“The notion of Korea Passing is not that new.
When Saudi government started referencing Korean pricing, the phenomenon occurred time to time.
But it became as big of an issue, because the impact it bring is comparatively more detrimental than before”, an industry insider explained.
“Especially after China officially added Korea as one of external reference pricing countries, the industry’s concern on Korea Passing has grown exponentially.
Figures may not yet show the impact, but the influence has been substantial”, they added.
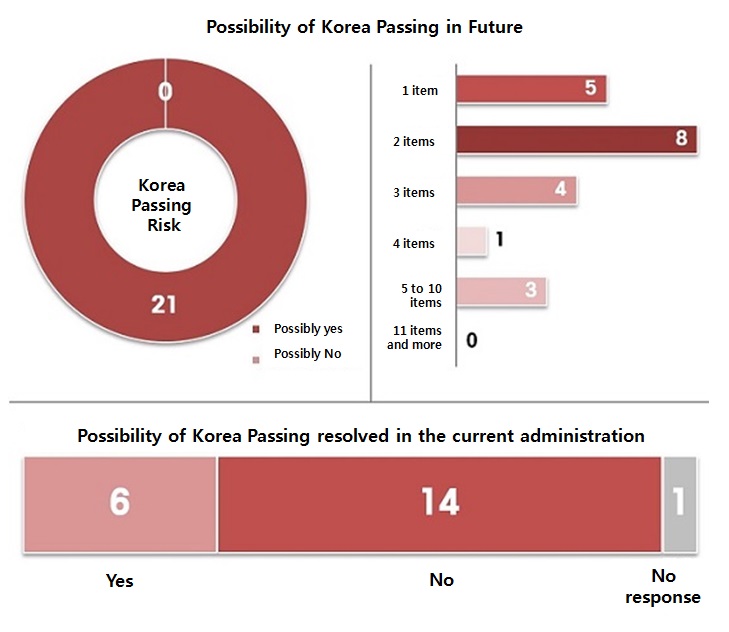
All agree “Worried about future releases in Korea” All of the survey participants had a same answer for one part.
They all agreed they currently have at least one item in risk of Korea Passing.
Eight or the majority of them said there are two items.
And it was followed by five answering one item, four saying three items, and one saying four, and three saying five to ten items.
None of them said ten or more.
Then what’s next for Korea Passing?
Questioning about their views on the current administration capable of solving the issue, 14 of them answered negatively.
Clearly, they are afraid of Korea Passing setting in over the time.
But, six said the otherwise.
Still some are positive the issue would get resolved, despite their serious concern about Korea Passing.
They are hopeful the phenomenon is a temporary occurrence, and the Korean government and pharmaceutical industry would find a solution.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









