- LOGIN
- MemberShip
- 2025-12-27 17:08:56
- Hanmi’s diabetes pipeline still standing in the storm
- by Chon, Seung-Hyun | translator Byun Kyung A | 2019-12-11 06:47:32
Hanmi Pharmaceutical’s efpeglenatide with the title of the biggest license-out deal to date is going through harsh times before reaching the finish line.
The company’s unbent commitment for the pipeline kept it alive, despite amending the license out agreement twice and the partner company shifting R&D pipeline focus.
Although it seems to have international clinical trials on their way unaffected, the company is now concerned of change in global commercialization partner.

10, Hanmi Pharmaceutical’s stock price closed at 298,000 won with 6.88 percent drop from the day before.
Hanmi Science also took a 4.57-percent fall.
Experts see that news of Hanmi Pharmaceutical’s partner company, Sanofi looking for a partner to launch efpeglenatide may have intimidated the investors.
◆Efpeglenatide surviving albeit Sanofi’s R&D pipeline shake-up In fact, efpeglenatide’s development timeline has not been changed.
Sanofi broke a news of acquiring a U.S.-based oncology R&D company Synthorx for USD 2.5 billion (approximately three trillion won) on Dec.
9, and elaborated its plan to shift the focus of R&D pipeline and management style.
Sanofi official stated it would prioritize investment on oncology, rare disease, blood disorder and neurology, while it would cease researches on diabetes and cardiovascular disease.
However, the multinational company’s news also included its plan to complete ongoing Phase 3 clinical trials of efpeglenatide, but not to pursue a efpeglenatide launch.
At face value, Sanofi means to not commercialize efpeglenatide, but it also means the pipeline survived another major pivot in Sanofi’s R&D pipeline.
The candidate drug is the only diabetes treatment pipeline among five investigational drugs the multinational company plans to submit the U.S.
Food and Drug Administration (FDA) New Drug Application (NDA) for in two years time.
A Sanofi executive stressed “It was the best decision for the successfully launch of efpeglenatide while maximizing the productivity of our research engine.
It was irrelevant to efficacy and safety of the substance, and it would make no changes on license-in agreement with Hanmi Pharmaceutical”.
Technically, efpeglenatide proved its potential to Sanofi’s new CEO, Paul Hudson appointed last August, despite his firm commitment to reprioritize R&D pipeline.
In the year, Sanofi suddenly stopped research in a trigonal GLP-1/GIPR/GCGR agonist, SAR441255.
And the French company also has paid back upfront fee to exit a 300-million-dollar partnership deal over SGLT1/2 dual inhibitor Zynquista (sotagliflozin) signed in 2015.
Efpeglenatide, a GLP-1 injection for type 2 diabetes, is a bio drug candidate that extended once-daily administration interval to once-weekly and even once-monthly.
It incorporated Hanmi Pharmaceutical’s key platform technology, ‘LAPSCOVERY’.
LAPSCOVERY, or Long Acting Protein/ Peptide Discovery platform technology prolongs the duration of biologics’ short half-life to reduce administration frequency and dose, which would ultimately reduce adverse reaction and improve efficacy.
Chemically conjugated biologics and protein ‘LAPS-carrier’ amplifies duration of the substance’ effect in human body and maintains the effect for maximum one month even with a small amount.
In November 2015, Hanmi Pharmaceutical signed a license-out agreement with Sanofi on efpeglenatide, which still to this date is the biggest deal in the history of Korean pharmaceutical industry.
The 2015 deal transferred technology of a EUR 3.9 billion-worth Quantum Project (efpeglenatide, long-acting insulin, and efpeglenatide with long-acting insulin) to Sanofi.
The upfront fee alone was 400 million euro.
But after closing the deal, a number of unexpected changes were made.
In December of 2016, Hanmi Pharmaceutical amended the agreement to drop one of the drug candidates from the ongoing technology transfer project.
Out of three drug candidates, Sanofi decided to return license over the long-acting insulin.
Agreement terms on the long-acting insulin combination was also changed so that Sanofi would acquire it after Hanmi Pharmaceutical develops it for a certain period of time.
As a result, Hanmi Pharmaceutical paid back 196 million euro out of Sanofi’s 400-million-euro upfront payment.
The milestone payments were also affected and the overall deal was shrunk by over one billion euro, down to 2.82 billion euro.
Regardless of the reduction, the upfront fee and overall scale of the efpeglenatide deal is still historic record high within the Korean pharmaceutical industry.
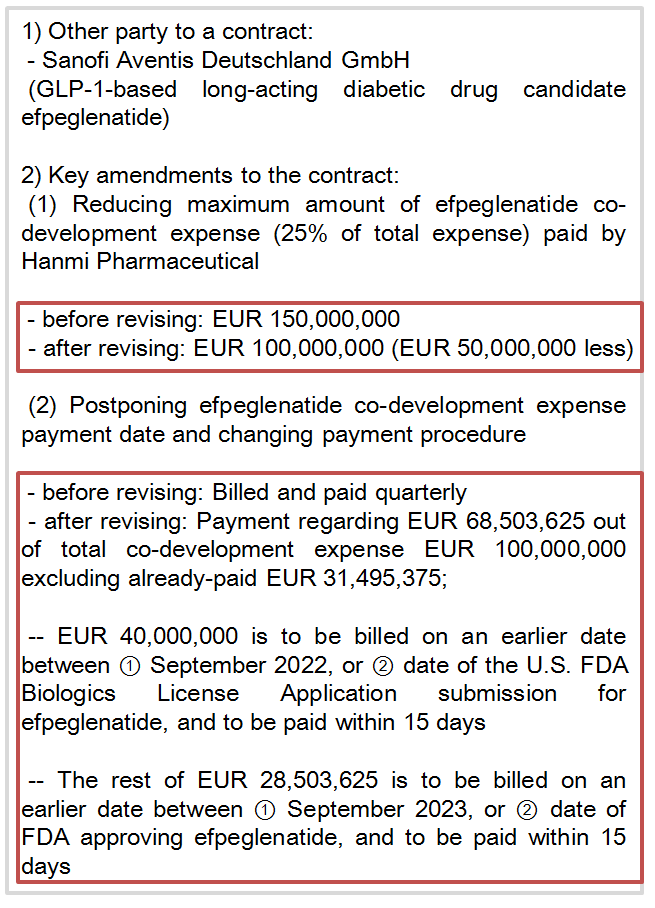
Hanmi Pharmaceutical lowered the maximum co-research expense from 150 million euro to 100 million euro with about 50 million euro (approximately 65 billion won) cut.
The first amendment actually included a clause for Hanmi Pharmaceutical to contribute 25 percent of efpeglenatide R&D expense, which was supposed to be covered entirely by Sanofi.
And Hanmi Pharmaceutical set the research expense cap at 150 million euro.
In just about two years, Hanmi Pharmaceutical was able to revise the clause added in 2016 to be more favorable to them.
When amending the agreement for the second time, Hanmi Pharmaceutical also postponed its payment period of clinical trial expense.
Initially, Hanmi Pharmaceutical was supposed to pay when Sanofi quarterly billed for efpeglenatide’s clinical expense.
The additional expense of 40 million euro out of 68.5 million euro would be billed on an earlier date between September 2022 and submission date of Biologics License Application (BLA) to FDA.
The bill is to be cleared by Hanmi Pharmaceutical within 15 days.
The rest of 28.5 million euro would be paid on earlier date, either in September 2023 or the FDA approval date.
Basically, Hanmi Pharmaceutical is to pay Sanofi whenever efpeglenatide development achieves a milestone.
When Sanofi initiated large scale clinical trials on efpeglenatide, the expense soared more than expected.
But then Sanofi seems to have accepted Hanmi Pharmaceutical’s request to lower its contribution in clinical expense.
Meanwhile, risk factors in LAPSCOVERY technology have been raised.
Janssen signed a technology transfer deal on JNJ-64565111, a candidate diabetic obesity drug, but it had to suspend clinical trial due to production delay.
In July, Janssen returned its license on JNJ-64565111 and the license-out agreement covering two candidate drugs based on LAPDISCOVERY technology fell through.
Spectrum Pharmaceutical’s in-licensed Rolontis, a neutropenia treatment, is the first LAPSCOVERY-applied medicine to get so close to commercialization.
A U.S.-based Spectrum Pharmaceutical filed for FDA approval on Rolontis at the end of last year, but it dropped the BLA in March as the authority ordered for more supplementary data.
The application was submitted again in October.
◆ Five out of two efpeglenatide clinical trials completed selecting participants Currently, the efpeglenatide development is going smoothly.
Two years after signing the deal, Sanofi officially unveiled a detailed plan on efpeglenatide at the end of year 2017.
By the end of 2017, Sanofi initiated the first Phase 3 clinical trial to compare efpeglenatide and placebo, and in April last year, the company initiated a large-scale Phase 3 trial to test efficacy and safety in treating cardiovascular diseases.
Another Phase 3 trial was initiated since September last year to compare efpeglenatide and competing product Trulicity (dulaglutide) as a combination therapy with Metformin.
In October last year, a protocol on combination therapy with efpeglenatide and basal insulin was registered, and in December same year, the fifth Phase 3 trial was initiated to confirm the drug treating Type 2 diabetic patients who cannot control glucose level either after Metformin single therapy or Metformin and sulfonylureas combination therapy.
The overall target number of sample is 6,340.
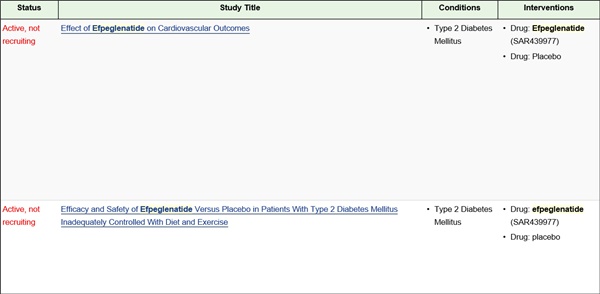
As of now, two out of five Phase 3 trials on efpeglenatide have gathered sufficient number of participants.
Most recently, placebo-comparing Phase 3 trial AMPLITUDE-M has gathered targeted number of patients.
The investigational drug’s key trial, AMPLITUDE-O testing effect of efpeglenatide on cardiovascular outcomes has registered more than planned number of participants of 4,076 patients in June.
A Hanmi Pharmaceutical official stated, “Sanofi has promised to concentrate on completing numerous Phase 3 trials currently ongoing to develop efpeglenatide successfully”.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









