- LOGIN
- MemberShip
- 2025-12-27 17:20:35
- MFDS to wait and see self-test results on metformin
- by Chon, Seung-Hyun | translator Byun Kyung A | 2019-12-13 06:34:58

Unlike the ranitidine and nizatidine incidents, the government means to wait and see the companies’ self-investigation results, rather than to take an action by itself.
Basically, metformin would be the first substance to be applied with the recently unveiled impurity risk management initiative.
However, the possibility still exists for the government to immediately call for sales ban and other follw-up measures, when a exceeding level of impurity is found in a product, either in Korea or overseas.
According to the industry source on Dec.
12, Korea’s Ministry of Food and Drug Safety (MFDS) has not made any official order to pharmaceutical companies regarding findings of cancer-causing impurity in metformin.
On Dec.
4, Singapore’s Health Science Authority (HSA) has decided to recall three out of 46 metformin products tested.
Apparently, the regulator found N-nitrosodimenthylamin (NDMA) exceeding an acceptable level of daily consumption in metformin products.
Since then, the U.S.
and European health regulators have also started probing on contaminated metformin products.
The U.S.
Food and Drug Administration (FDA) is reportedly investigating NDMA discovered in metformin products available in the U.S.
It also stated it would recommend recalling products with exceeding levels of NDMA contamination.
The European Medicines Agency (EMA) has ordered companies to conduct NDMA contamination testing.
Japan’s Ministry of Health, Labor and Welfare recently ordered metformin containing product manufacturers and importers in Japan to analyze and report risk of NDMA contamination in the substance and complete products.
So far, MFDS has only stated it would “review investigation results”, and no other specific orders have not been given to the companies.
The ministry has not yet ordered the companies to investigate NDMA in metformin active agent or complete product, nor has it stated the ministry would directly collect and investigate the substance.
The ministry’s reaction is contrasting from the follow-up measures it had with other pharmaceutical impurity risk incidents.
When the European regulator announced it would recall all valsartan products, the Korean ministry immediately banned sales of products with the same active ingredient.
And after the sales ban was ordered in the U.S., the ministry once said “nothing to worry about”, but soon after it ordered an all-product ban.
Actions on nizatidine followed the ranitidine sales ban.
After collecting and testing active ingredient and complete product with similar chemical structure as ranitidine, MFDS banned sales on 13 items with unacceptable level of NDMA.
It could be interpreted that the authority is trying to encourage companies to voluntarily test their metformin products.
Neither of the U.S.
or Europe has called for a recall due to exceeding level of the contamination.
And the complete products reported with the impurity to date are not available in Korea, yet.
MFDS official explained, “We have already asked companies to test the impurity in all products themselves”.
It also means the recently introduced active ingredient impurity risk management initiative would be applied on metformin.
In fact, MFDS has already asked pharmaceutical companies to run NDMA-like impurity test on all synthetic active agents, when announcing the investigation result of nizatidine medicine.
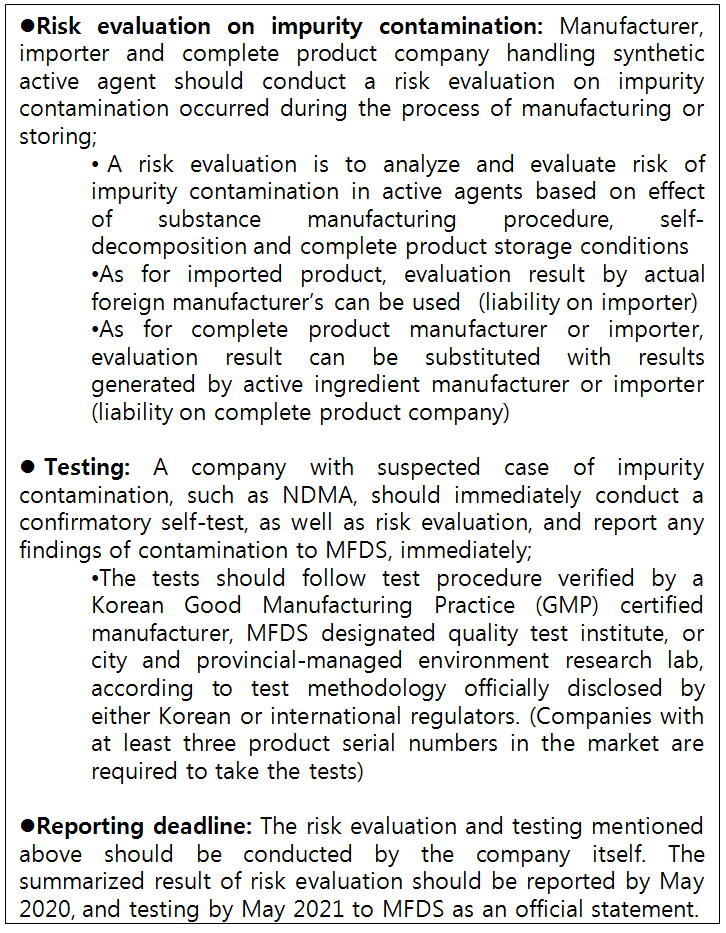
Accordingly, synthetic ingredient manufacturer and importers, as well as complete product companies, have to evaluate risk of impurity contaminated during the process of manufacturing or storing.
Pharmaceutical companies suspecting a risk of discovering NDMA and other harmful impurity in their product should immediately conduct a voluntary test and has to report MFDS as soon as possible when it is actually found.
The impurity risk evaluation and testing should be conducted voluntarily, and the result of risk evaluation should reported by May 2020, and the result of test by May 2021.
In other words, MFDS is unlikely to test metformin itself, as of now.
Moreover, metformin being a commonly used agent seems to make MFDS hesitant about testing the products proactively.
Metformin is the most commonly prescribed first-line treatment for patients with Type 2 diabetes to control glucose level.
In Korea alone, 642 of approved complete products contain metformin.
Practically, all pharmaceutical companies have at least one metformin medicine.
Compared to valsartan and ranitidine market, metformin has a far larger market volume.
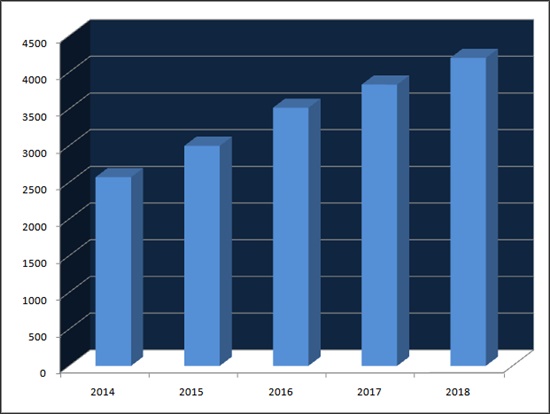
Pharmaceutical industry research firm UBIST reported outpatient prescription market volume of metformin product reached 420 billion won last year.
It soared 63.4 percent in four years from 257.1 billion won in 2014.
Currently banned ranitidine products generated about 200 billion won as outpatient prescription, but metformin market easily doubles the ranitidine market.
A single dose of metformin is priced at less than a hundred one with reimbursement.
Considering the pricing, metformin’s usage volume is overwhelmingly bigger than ranitidine.
In such case, millions of serialized products would have to be investigated.
It is physically impossible for MFDS to directly investigate them all.
However, the ministry may initiate a full investigation, depending on the updates from home and abroad.
The ministry’s hands-on investigation would be inevitable when active ingredient or complete product in Korea is found with exceeding level of impurity is reported from overseas.
And even when a Korean company reports any case of NDMA contamination exceeding an acceptable level, full investigation is likely follow.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









