- LOGIN
- MemberShip
- 2025-12-27 17:20:36
- Industry feels growing pressure on metformin risk
- by Chon, Seung-Hyun | translator Byun Kyung A | 2019-12-16 06:21:22

The industry’s eyes are focused on the government’s action following its ongoing metformin usage status review.
Some of pharmaceutical companies are promptly equipping themselves with impurity analyzer a self-testing.
◆MFDS initiates metformin usage status review, the industry fears of follow-up action An industry source reported on Dec.
15 that Korea’s Ministry of Food and Drug Safety (MFDS) has ordered pharmaceutical companies to assess manufacturing record of pharmaceutical product containing ‘metformin hydrochloride’ and investigate used active pharmaceutical ingredient.
MFDS asked companies to report total number of drug items consisting of metformin substance, names and number of manufactured items, and names and number of non-manufactured items until Dec.
17.
For example, when an items’ use-by date is three years, then the item’s entire manufacturing record after December 2016 should be looked into.
The ministry is also calling for a full report on active ingredients used in the metformin products.
It means the ministry means to collect detailed information on DMF registration number and manufacturing plant of a complete product containing the ingredient under each serial number.
The procedure resembles the ranitidine and nizatidine cases.
When they find an issue in specific active ingredient and complete drug, the ministry can take a fast and accurate action based on the information submitted by companies.
The pharmaceutical industry predicts the government direction is a part of the ministry’s preparation process, in case impurity is discovered in metformin medicine.
Before the government body took an action on ranitidine and nizatidine cases, the ministry had reviewed detailed information on complete product and the active ingredients.
MFDS had immediately ordered a sales ban on drugs with valsartan when Europe decided to recall the products, but it held back on the action for ranitidine and nizatidine until the thorough review on complete products and the ingredients was completed.
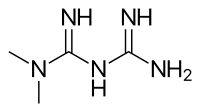
The risk of metformin impurity broke out from Singapore.
On Dec.
4, Singapore’s Health Sciences Authority (HSA) recalled three items out of 46 metformin containing drugs they investigated.
The result confirmed contamination of N-Nitrosodimethylamine (NDMA) has surpassed daily acceptable level.
Since then, the U.S., Europe and Japan initiated impurity testing on metformin drugs.
All three regions are recommending companies to conduct self-testing on NDMA levels in their metformin drugs.
◆Companies initiating impurity self-tests and purchasing analyzer Companies in Korea are also encouraged to run self-tests on their metformin-containing items.
So far the ministry has not officially instructed companies to test impurity in metformin ingredients.
In fact, the ministry has not even ordered them to test NDMA level in complete product or announced a plan to collect all items for further investigation.
MFDS’ lack of instruction could be because of the U.S.
and Europe have not ordered for a recall on products with alarming impurity level, and also because the problematic metformin ingredient and complete product with the ingredient have not even been imported to Korea.
Moreover, there has not been an official procedure of NDMA testing presented by the ministry.
The complete products with contaminated metformin recalled in Singapore have not been imported to Korea, yet.
However, it has not been confirmed yet if the active ingredient used in the recalled product has been used in Korea.
Apparently, the Singaporean regulator has decided to recall the products after testing complete products, not the active ingredient.
The pharmaceutical companies in Korea have no other choice, but to test their metformin products according to the active pharmaceutical ingredient impurity risk management measure the ministry has unveiled recently.
While announcing the investigation result of nizatidine contamination last month, MFDS ordered companies to conduct a self-test on all of their synthetic active ingredients for impurity like NDMA.
Accordingly, synthetic ingredient manufacturer and importer, as well as complete product companies, have to evaluate contamination risk in drugs either during manufacturing or storing processes.
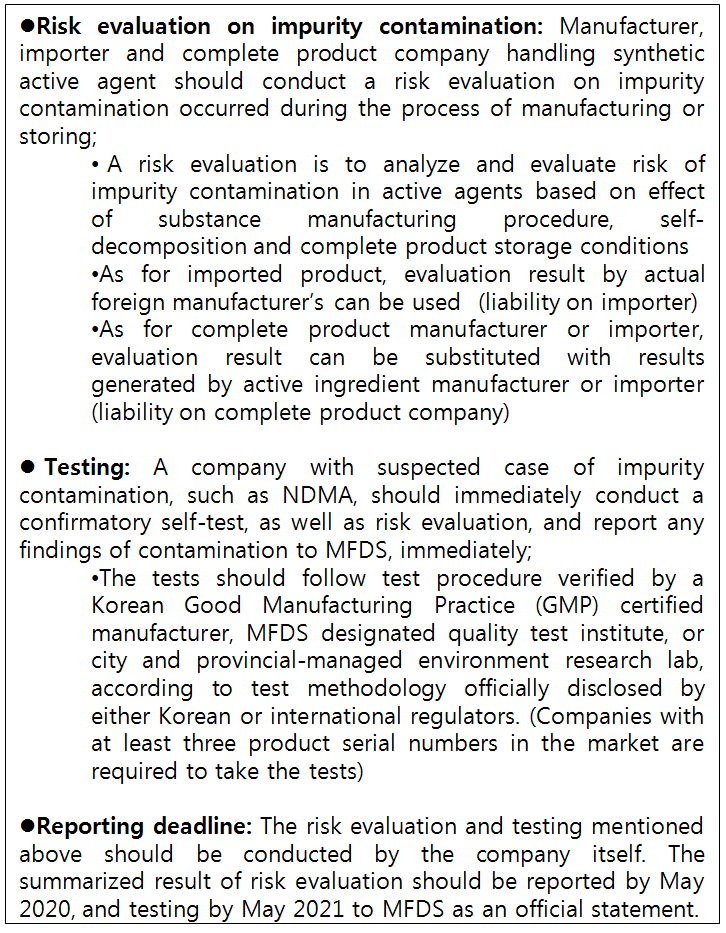
Basically, metformin became the first substance to be ‘suspected for contamination risk’ since the ministry’s impurity risk management initiative.
The industry seems to be in process of attempting self-analyze NDMA level in metformin medicine.
However, the testing process has not been so simple without an official testing procedure and lack of laboratories with proper analysis equipment.
Currently, only nizatidine-containing products verified with approved level of NDMA can be distributed, but the testing laboratories are unable deliver testing results in requested time as they are backed-up with all companies with nizatidine products.
MFDS has ordered pharmaceutical companies to supply nizatidine-containing item with passing level of cancer-causing NDMA contamination (less than 0.32ppm), verified by testing done for each item serial number.
The test can be done by Korean Good Manufacturing Practice (GMP) certified manufacturer, MFDS designated quality test institute, city and provincial-managed environment research lab and Korean Pharmaceutical Traders Association.

But an analyzer costing up to 300 million to 500 million won, beside the six-figure annual maintenance, cost is definitely putting a strain on the company.
A pharmaceutical company insider commented, “The company is taking account of equipping impurity risk examination system as its own quality management program.
We are also discussing possibility of sharing an ownership and purchase cost of the analyzer”.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









