- LOGIN
- MemberShip
- 2025-12-27 17:20:36
- Champix, conclusion of patent disputes imminent
- by Kim, Jin-Gu | translator Choi HeeYoung | 2019-12-17 06:34:00

The Patent Court has announced that it will issue a judgment on a patent dispute related to the Champix on the 20th , 4 days later.
The result of the Champix patent dispute attracted interest in that it could see the future of domestic salt-modifying drugs after the so-called ‘Solifenacin decision’ earlier this year The legal disputes between the two sides were so intense that the court would postpone the sentence four times.
The ruling, originally scheduled for February, was postponed for four months, from May to August to October to December.
The jurisdiction of the judiciary is also a matter of how deep.
The ruling of the patent court is expected to be applied as a definitive ruling.
This is because the Supreme Court's appeal is unlikely, as the expiration date of the material patent is almost imminent.
◆Patent Judge sided with generic company, Pfizer appeals In September 2016, more than 20 domestic companies, including Hanmi Pharmaceuticals, filed a patent dispute with Pfizer.
Domestic companies tried to speed up the launch of generics with the strategy of “avoiding patents with salt changes.” It is a strategy that avoids the extended duration of material patents by developing salt-changing generics and raising passive judgments on the scope of rights.
The Patent Judge, the first case of a patent dispute, raised the hands of domestic companies.
Based on this, domestic companies have launched salt-modifying drugs since last November.
Pfizer dissatisfied with the decision of the Judge.
The company filed a lawsuit with the Patent Court asking to cancel the decision.
The ruling on the lawsuit is sentenced on the 20th.
◆Interpretation over Supreme Court's decision to Solifenacin. During the second trial over Champix, a ruling was imposed elsewhere that would have a modest impact on the case.
It was a Solifenacin decision issued by the Supreme Court in January this year.
The Supreme Court sided with the original company, Astellas, in a salt-altering patent dispute between the original company and the generic company over Solifenacin, an overactive bladder treatment.
The key point of the ruling was that ‘the salt-modified product and the original product had substantially the same active ingredients, therapeutic effects, and uses, and those skilled in the art could easily change the salt’.
Interpretations were mixed with this ruling.
Pfizer and other originals interpreted the Supreme Court as setting a case for the salt change, which has been the main patent evasion strategy of domestic companies.
Of course, the case is expected to affect the results of the Champions suit.
On the other hand, some domestic companies draw a line that the case of Solifenacin salt-modifying drug and the Champix salt-modifying drug, which were previously lost, is quite different.
They explain that the 'substantial identity' that has been crucial in the Solifenacin decision is a separate part of the decision.
They also argues that there is a difference in the ease of realization of technology by trade technicians.
◆If a domestic company loses, 'Change salt patent avoidance' strategy useless

If the patent court raises Pfizer's hand in accordance with the Supreme Court case, domestic companies will not be able to use the patent evasion strategy by changing the salt.
On the other hand, if the patent court raises the hands of domestic companies separately from the Soliphenacin case, the avoidance of salt-changing patents by domestic companies is likely to continue strategic vitality.
A legal personnel said, “At present, the patent court is expected to apply Solvenasin's judgment as a precedent and side with Pfizer, however, as domestic companies insist, there is some possibility of judging the Solifenacin case and the Champix case separately.
Either way, it will have a big impact on our strategy for avoiding salt modifications in the future”.
◆Material patent expires after 7 months, Less possibility of 3 trial appeals On the other hand, even if the patent court raises Pfizer's hand, there is a possibility that domestic companies are unlikely to lead the case to the Supreme Court.
This is because the champix material patent expiration is shortly until July 19 next year.
Given the time it takes to appeal after the Supreme Court appeals, it is likely that the avoidance of the extended patent duration, which was the original purpose of the patent dispute, has already been achieved.
In addition, as domestic sales of Champix have been plunging recently, domestic companies' economic incentives have weakened significantly.
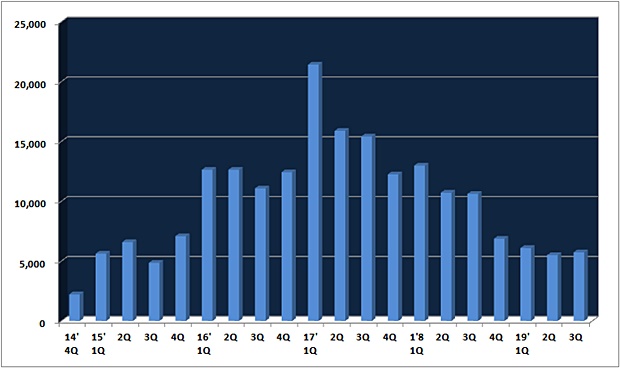
According to drug research firm IQVIA, Champix's 3Q revenue was ₩5.7 billion, down 46% from ₩10.6 billion a year earlier.
It is a quarter compared to the peak season, ₩21.4 billion in the first quarter 2017.
An official in the pharmaceutical industry said, "With a huge drop in sales of successful successes, we know that many pharmaceutical companies have decided to stop the release of Champix salt-modifying drugs regardless of the Soliphenacin case."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









