- LOGIN
- MemberShip
- 2025-12-27 15:26:30
- New flu season to spark up another round of competition
- by Kim, Jin-Gu | translator Byun Kyung A | 2019-12-18 14:25:15

Korea Centers for Disease Control and Prevention (KCDC) issued a flu warning on November 15.
The season is to last until May next year.
The new flu season is to start up even more fierce competition in the flu treatment market.
The market leader, Tamiflu (oseltamivir) sales has been tumbling down since oseltamivir generics were launched.
What’s worse, the flu treatment’s adverse reaction risk reported last season had its market position precarious.
Now the gap between Tamiflu and the runner-up Hanmi Flu is in arm’s length.
Prescription volumes of generics, on the other hand, are neck-and-neck, while the original’s performance is stagnating.
Moreover, intravenously administered Permiflu jumped in to the already-heated competition strong with convenience of single-dosage.
Now with the new flu season just penning, let us round up the highlights of the flu treatment market.
◆Tamiflu adverse reaction risk from last year, so what’s next this year?
Last winter, Tamiflu was struck down with a concerning issue.
In December last year, a middle school student fell from an apartment and was found dead in Busan, after taking Tamiflu.
The student’s family suspected adverse reaction as she complained of hallucination after taking Tamiflu.
And the media highlighted sporadic reporting of suicidal reaction from Tamiflu in Japan.
The risk did not subside easily.
As a result, the original and oseltamivir generics together generated prescription volume of 24.7 billion won in last flu season from November 2018 to May 2019.
Compared to the 2017-2018 season generating 31.1 billion won, the last season’s sales dropped more than 20 percent.
Besides, it was a big loss for the drugs considering the flu season struck hard last year.
According to Health Insurance Review and Assessment Service (HIRA), 4.74 million patients visited hospitals for flu in the 2018-2019 season.
The figure was at four-year high and it was 5.5 times more than 860,000 patients in the 2014-2015 season and 2.1 times more than 2.29 million patients in the 2017-2018 season.
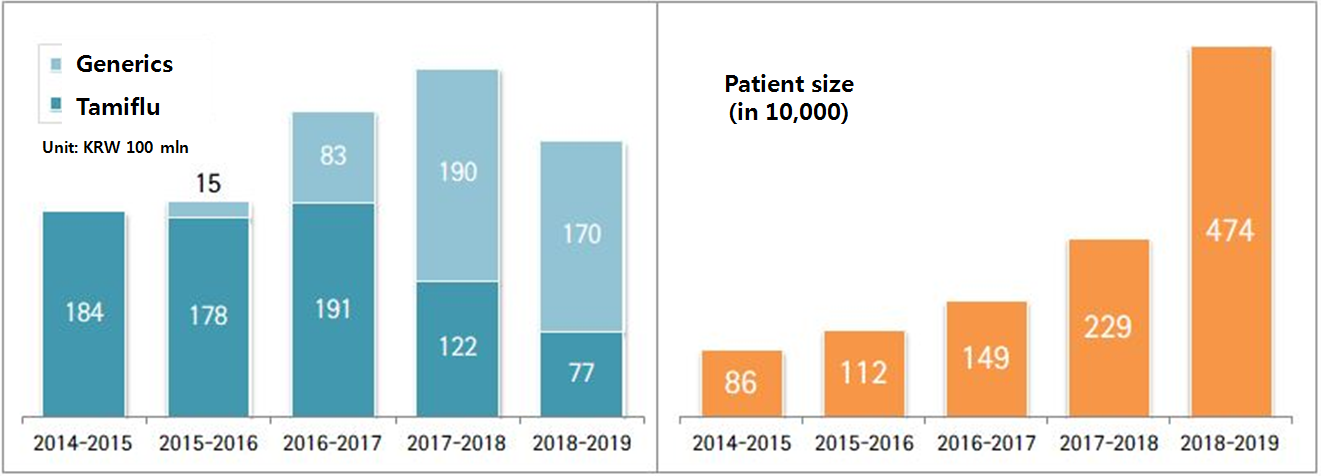
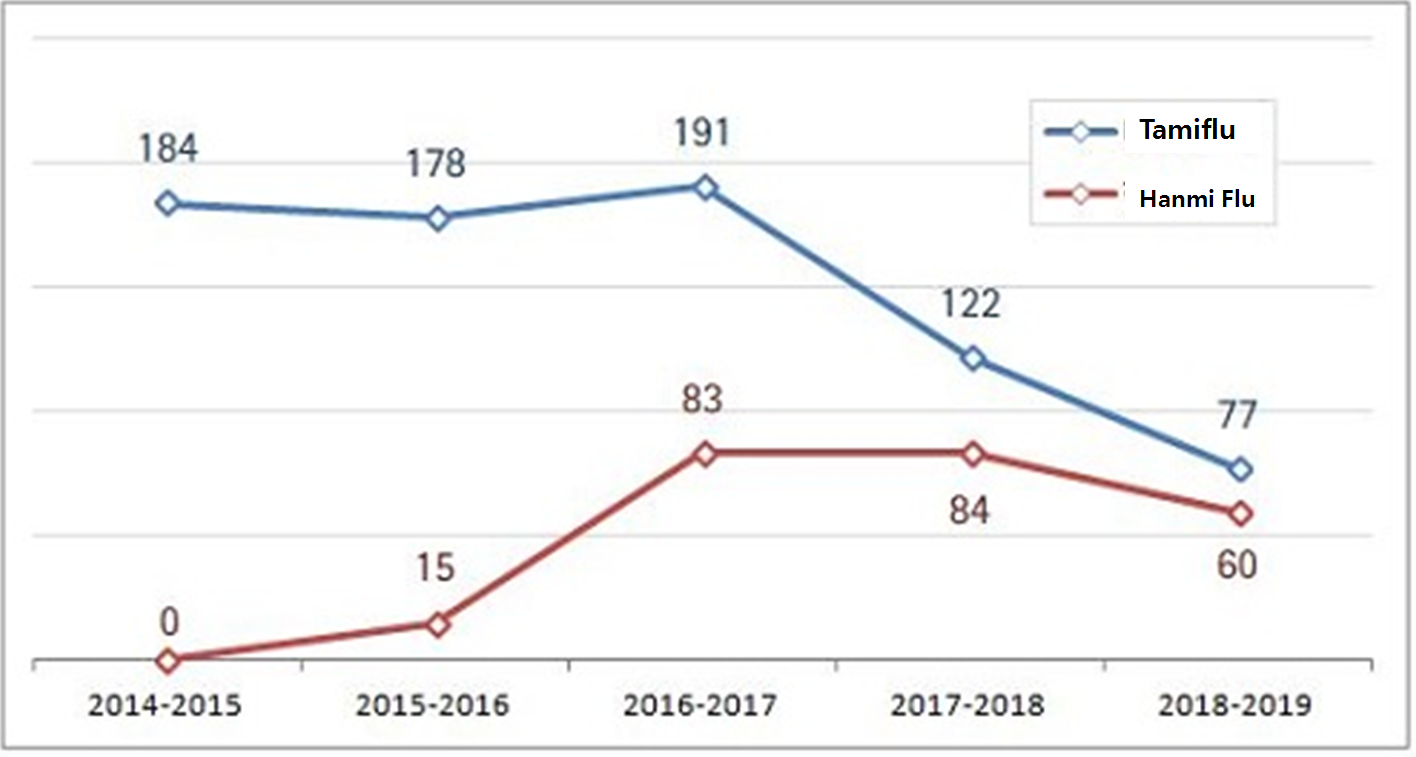
Its prescription volume plunged from 12.2 billion won to 7.7 billion won in a year.
Hanmi Flu generating the second highest sales narrowed the gap with Tamiflu from 3.8 billion won to 1.7 billion won in the same period.
The industry predicts the generic could actually surpass the original this season according to the trend.
Compared to the previous season, the peramivir medicine’s market share more than doubled last season.
The National Assembly Health and Welfare Committee reported 67,518 patients were prescribed with Peramiflu as of December last year.
It was 4.4 times more than 15,481 patients in the season before.
In the general flu treatment market including Tamiflu, Peramiflu’s share soared from 2.0 percent to 5.4 percent, about 2.7 times the rate.
Some hospitals and clinics struggled to get a hold of Peramiflu stock, because a sudden spike in sales exceeded projected demand,.
Apparently, pediatric institutes especially had difficulty in acquiring needed supply.
Peramiflu also won expanded indication on pediatric patient under the age of two.
And some sees that the word of mouth from young patients’ parents, concerned of the recent Tamiflu risk, have affected the shortage.
◆Hanmi Flu gulping up generic market share Since Roche introduced Tamiflu to Korea in 2000, its market share grew significantly in 2009 with the new influenza sweeping the nation.
And the treatment dominated the market in the 2014-2015 season.
In the 2015-2016, Hanmi Pharmaceutical modified the original’s saline substance to avoid patent infringement and launched Hanmi Flu.
The incrementally modified drug generated 1.5 billion won from the first season’s prescription and had a perfect soft landing on to the flu treatment market.
The prescription sales also hiked exponentially in the 2016-2017 season at 8.3 billion won.
From the 2017-2018 season, Tamiflu’s patent was expired and generics flooded out to the market.
About 50 pharmaceutical companies entered the oseltamivir generic market.
The generics made a huge success from their first year.
31.1 billion won worth of generics were prescribed in their first flu season from 2017 to 2018.
But the upside was that the market share of generics in the overall flu treatment market surged from 60.9 percent to 68.8 percent.
The generic’s share is expected to surpass 70 percent this season.
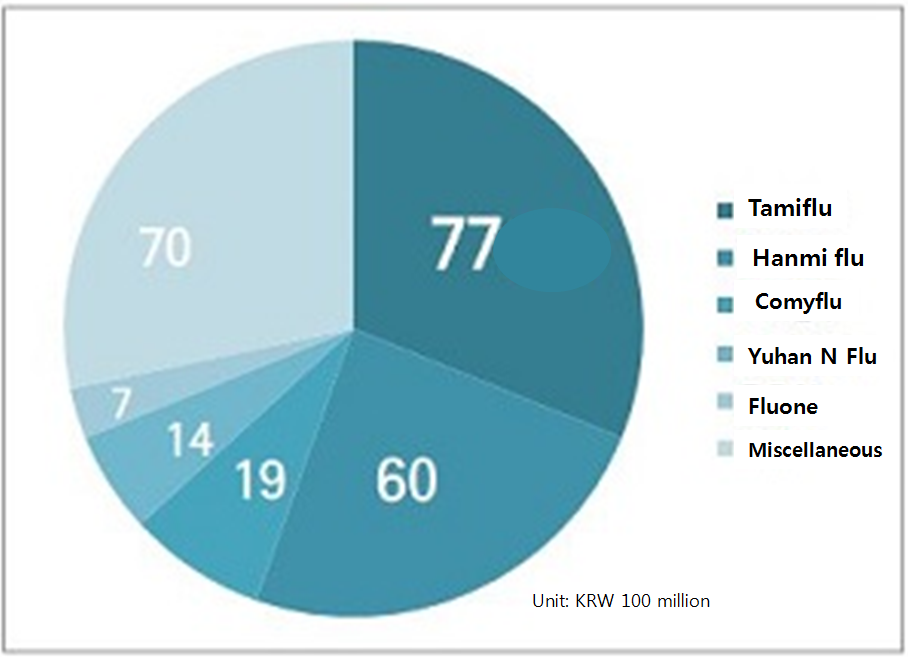
Interestingly, the number one generic Hanmi Flu’s prescription volume has dropped from 8.4 billion won in 2017-2018 season with share of 26.9 percent to 6.0 billion won in 2018-2019 season with 24.3 percent market share.
The decrease in Hanmi Flu’s market share was actually shared among other generic items including Fluone (Jeil Pharm), Seltaflu (Inist Bio), Tami-infle (Hutecs), Boryung Tami (Boryung Pharmaceutical), Tamipro (Arlico Pharm), Bisel Flu (Wooridul Pharmaceutical), Dongwha Flu N (Dongwha Pharm) and Tamiforce (Ilsung Pharmaceuticals).
All of the said generics had growth in sales and market share.
Meanwhile, Roche Korea is aiming for a turnaround with a new launch of Xofluza.
However, the company would be unlikely to recover the fall this season as the new flu treatment launch would take a place in March next year at earliest.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









