- LOGIN
- MemberShip
- 2025-12-27 13:39:16
- Anti-obesity drug market stirred by lorcaserin safety issue
- by Jung, Hye-Jin | translator Byun Kyung A | 2020-01-22 06:27:55

Lorcaserin competitors are also getting busy anticipating doctors and pharmacists to avoid Ildong Pharmaceutical’s Belviq (locarserin), which was considered a safe anti-obesity drug.
However, the industry experts note the lorcaserin safety issue would not significantly affect the anti-obesity drug market, as it has been generally stagnant except for Saxenda.
According to pharmaceutical industry sources on Jan.
19, a pharmaceutical company in Korea supplying an anti-obesity medication has ordered their salespeople to collect information of Belviq-prescribing hospitals and clinics in their designated regions.
The company seems to try using Belviq’s safety issue to leverage sales of their own anti-obesity drug.
The safety warning notice on Belviq could be game changer in the anti-obesity drug market, because the competition is saturated with Alvogen Korea, Huon, Kwang Dong Pharmaceutical, Chong Kun Dang Pharmaceutical, as well as Ildong Pharmaceutical.
Ministry of Food and Drug Safety (MFDS) have issued a notice on Jan.
17 advising pharmaceutical experts to consider if Belviq’s benefit outweighs the potential risk after the U.S.
Food and Drug Administration’s (FDA) warning issued on Jan.
14 about possibility of Belviq causing cancer.
Although a direct correlation of Belviq causing cancer has not been fully investigated, the Korean ministry warned doctors and pharmacists to consider the risk of cancer when prescribing the drug as a clinical trial testing safety of the drug found issues.
MFDS plans to review clinical data and change indications on Belviq.
Since the launch in February 2015, Belviq has attracted a huge attention.
The anti-obesity drug market did not have a sufficient option after sibutramine was dropped out of the market due to adverse events.
Approved for Korean market in February 2015, obesity-treating Belviq was developed by the U.S.-based Arena Pharmaceutical and has been supplied by Ildong Pharmaceutical in Korea.
First time after 13 years, FDA cleared the drug as a weight loss treatment.
After signing an exclusive marketing deal in 2012, Ildong Pharmaceutical has conducted clinical trials for two years with Arena Pharmaceutical.
In the first year of launch, Belviq generated 13.6 billion won and topped the anti-obesity drug market.
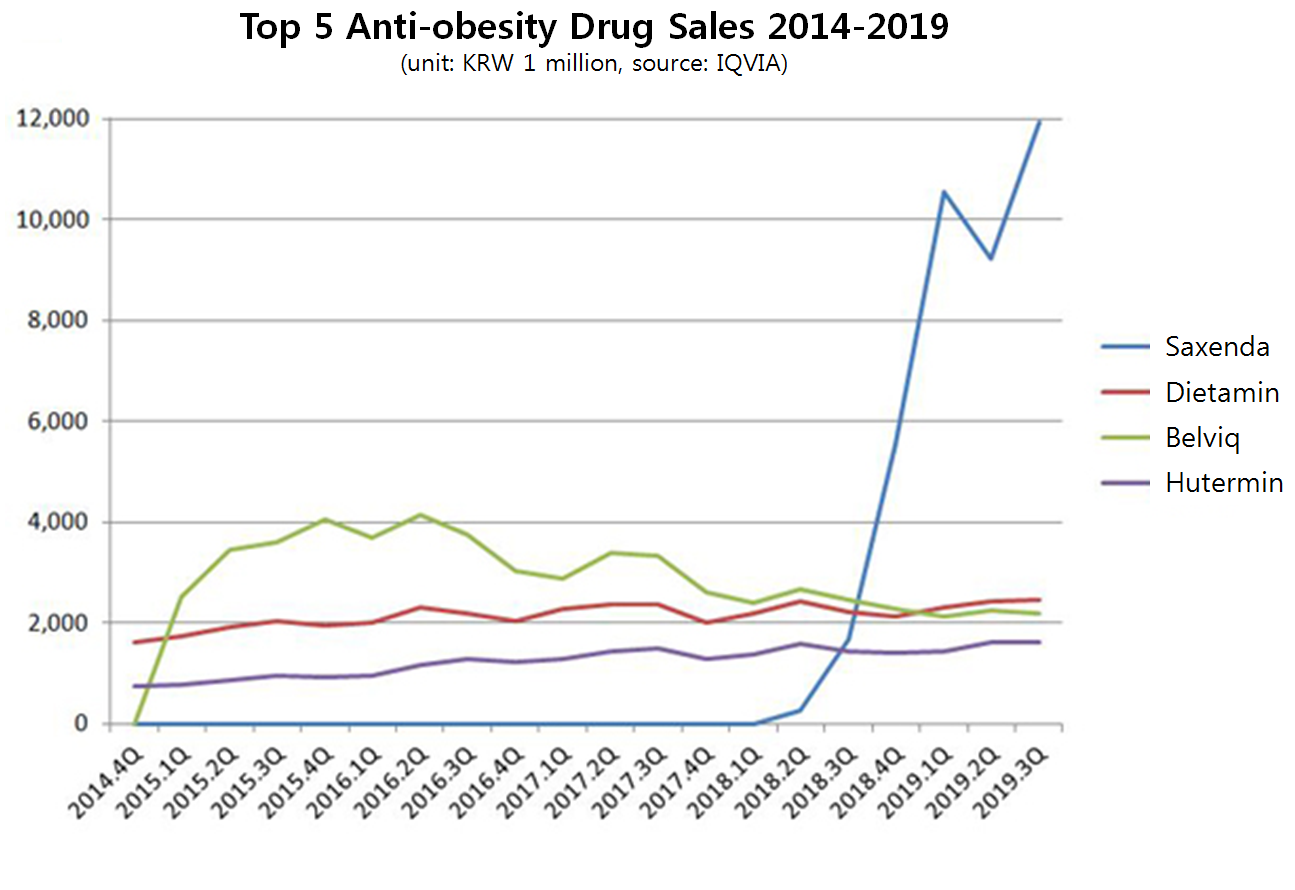
Belviq sales peaked in the second quarter of 2013 making 4.1 billion won but started descending right after.
Its growth was blocked by launch of other competitors like Kwang Dong Pharmaceutical’s Contrav and Novo Nordisk’s Saxenda.
In last third quarter, Belviq generated 2.2 billion won according to IQVIA.
It took an 11.8 percent fall compared to 2.5 billion won made in same period the year before.
Meanwhile, the anti-obesity medication market has been growing exponentially.
IQVIA reported, the market volume marked 97.0 billion won in 2018.
The overall volume in 2019 is expected to surpass 120 billion won as the market made 32.3 billion won, 33.1 billion won and 35.4 billion won in the first, second and third quarter last year, respectively.
The exponential growth in the anti-obesity drug market was mostly led by Saxenda.
The drug generated 11.9 billion won in the last third quarter, a seven-fold of what it has made in the same time previous year.
The third quarter sales was a five-fold of Dietamin’s sales, the second in the market.
Saxenda accumulated sales marked 32.0 billion won up to the last third quarter.
It took the first place in the market right when it was launched and has been widening the gap with the second top seller in the market.
On the other hand, the rest of the products’ market shares have been shrinking.
Belviq has been on the second place ever since Saxenda was released, but Dietamin took it over in the fourth quarter of 2018.
Contrav, slowly absorbing Belviq’s pie since its release in 2016, did not even sell over one billion won last quarter.
Except for Saxenda, the anti-diabetic drugs—Belviq, Dietamin, Hutermin, Furing, Fendy, Xenical, Contrav, Adipex, and Furimin—are fluctuating in the market ranking without much of difference.
Accordingly, experts do not expect one of them having a safety to cause a big shift in market.
Also, the experts’ prediction of the safety issue unaffecting the market sounds more credible as the uncertainty in the safety notice cannot conclude a major shift in market.
A pharmaceutical company insider elaborated, “As some of anti-obesity drugs are categorized as psychoactive drugs, not all pharmaceutical companies would take aggressive marketing strategy.
Because Saxenda has prominent market presences, it is highly likely that a safety notice on Belviq would shake up the market.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









