- LOGIN
- MemberShip
- 2025-12-27 10:20:45
- Sales activities messed up by COVID-19 crisis
- by Chon, Seung-Hyun | translator Choi HeeYoung | 2020-02-26 06:29:59

Concerned about COVID-19 infection, people are reluctant to visit medical institutions, and their business activities for medical staff are virtually suspended.
According to UBIST on the 25th, the total outpatient prescription amount last month was ₩1.25 trillion, down 4.4% from the same period last year.
It was 4.6% lower than last month.
The January prescription amount is unusual compared to the annual increase of more than 5%.
Last year's prescriptions increased 8.5% compared to January 2018.
In January 2018, prescriptions were up 18.2% year-on-year.
In 2016 and January 2017, prescription amounts increased 5.4% and 7.6%, respectively.

However, the analysis predominates that COVID-19 may have been the biggest factor in reducing prescriptions.
After the first COVID-19 confirmed in Korea last 20th, the alert for new infectious diseases has increased in earnest.
Since then, patients have been reluctant to visit medical institutions.
Indeed, prescriptions amount for major drugs sold heavily through outpatient prescriptions declined.
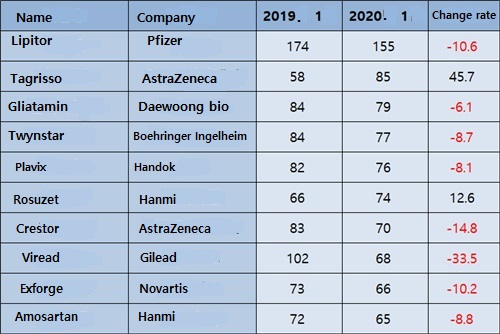
Pfizer's Lipitor, ranked No.1 in prescriptions, fell 10.6% YoY to ₩15.5 billion.
Daewoong Bio's brain function improver 'Gliatami' decreased 6.1%.
Over the past month, prescriptions for large-sized medicines, such as Twynsta, Plavix, Crestor, Viread, Exforge and Amosartan showed a steady decline compared to the previous year.
The outpatient prescription amount of AstraZeneca's anti-cancer drug Tagriso increased by 45.7% over the past month.
Presumably, this is because the proportion of patients who were taken at home while avoiding hospitalization increased.
The situation has worsened since the sudden increase in COVID-19 confirmers since the 19th.
COVID-19 spread rapidly in Daegu, Gyeongbuk, and more than 900 confirmed as of 25th.
On the 23rd, the government upgraded the COVID-19 crisis alert to the highest level of 'serious'.
The government issued a serious step only 11 years after the swine flu crisis in 2009.
Patients with mild illnesses, except those with severe or chronic illnesses, are reluctant to visit medical institutions because they are concerned about infection with COVID-19.
The drop in the number of patients leads to a decline in drug sales.
Pharmaceutical companies' sales activities are actually “open without any business”.
At the end of last month, telecommuting of sales staff, which was adopted by multinational pharmaceutical companies, is spreading.
Hanmi Pharm, LG Chem, GC Pharma, CJ Healthcare, Dong-A ST, and Dong-wha Pharm have banned sales people from visiting medical institutions.

There are also concerns that salespeople can be a powerful carrier if they are infected with COVID-19.
If there are confirmed patients among pharmaceutical salespeople, the company can not avoid blaming for spreading COVID-19.
While allowing salespersons to visit medical institutions, criticism may have been made that they exposed the risk of infection to COVID-19 and caused damage to medical institutions.
The Korean Medical Practitioners Association also sent an official letter to the Korea Pharmaceutical and Bio-Pharma Manufacturers Association on the 20th requesting that sales representatives refrain from visiting.
Promotional events such as product briefings are also being canceled.
An official of a pharmaceutical company said, “We are forced to cancel a pre-scheduled meeting or briefing session for physicians and pharmacists because of concerns about the infection of COVID-19”.
In fact, face-to-face sales activities have ceased.
Some pharmaceutical companies expect to benefit from masks and some over-the-counter drugs with COVID-19, but most of them are concerned about sales impact because their major businesses are Prescription drugs.
Disruption of business activities is inevitable for domestic pharmaceutical companies that are highly dependent on generics.
Sales of generic drugs with the same product power depend on their sales force.
Innovative new drugs with no alternative drugs or new drugs without generics are unlikely to hurt sales.
For pharmaceutical companies, they suffered from poor performance in the fourth quarter of last year due to an impurity issue.
The Ministry of Food and Drug Safety banned the sale of all anti-ulcer drug Ranitidine at the end of last September.
The market exit was virtually determined for the detection of carcinogen N-nitrosodimethylamine (NDMA) excess.
Pharmaceutical companies with ranitidine-based products suffered significant losses from sales bans, recalls, and disposals.
GC Pharm, Jeil Pharm, JW Pharma, and Ildong Pharmaceutical recorded losses in the fourth quarter of last year.
An official of a pharmaceutical company said, “The spread of COVID-19 has effectively stopped sales activities, and it is inevitable that there will be a considerable impact on performance, and in order to minimize prescription drug sales gap, there is a lot of concern in preparing effective marketing strategies in addition to traditional sales activities”.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









