- LOGIN
- MemberShip
- 2025-12-27 10:20:37
- 86% of generics for erectile dysfunction are domestic
- by Chon, Seung-Hyun | translator Choi HeeYoung | 2020-03-05 06:33:38
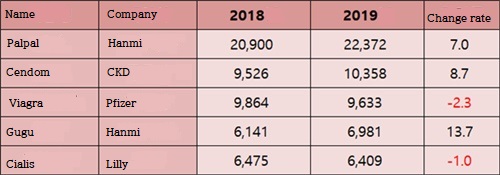
The sales rate of generic products developed in Korea in the erectile dysfunction market is very high.
Hanmi pharm's Palpal is one of hottest items, and Chong Kun Dang's Cendom continued to rise.
Domestically developed drugs account for 86% of the erectile dysfunction drug market, and the influence of domestic products is expanding.
According to IQVIA, a drug research agency on the 2nd, the market for erectile dysfunction drugs last year was 5.3%, up 5.3% year-on-year.
It is growing continuously every year, from ₩98 billion in 2016 to ₩104.5 billion in 2017 and ₩108.2 billion in 2018.
Hanmi's Palpal still showed its unrivaled position in the market.
Palpal's sales last year were up to ₩22.4 billion, an increase of 7.0%.
In the overall market for erectile dysfunction drugs, Palpal's market share is nearly 20%.
Palpal, which was released shortly after Viagra's patent expired in 2012, sells most since beating Viagra in 2013 and Cialis in 2015, respectively.
Considering that Palpal is less than half the price of Viagra, it can be calculated that the actual sales volume is more than four times.
In recent years, Chong Kun Dang's Cendom (generic for Cialis) sells well.
Cendom sold ₩10.4 billion, up 8.7% from last year.
Among the total erectile dysfunction treatment products, Cendom is the only product that has exceeded annual sales of ₩10 billion.
Cendom, which was released after the Cialis patent expired in September 2015, gradually surpassed Cialis in 2017 as its market share gradually increased.
Last year, it was overtaking even Viagra.
Gu gu's sales reached 4th place last year, reaching ₩7 billion, an increase of 13.7% over the previous year, and it beated Cialis for the first time since release.
Three generics including Palpal, Cendom, and Gugu, accounted for more sales than the original drugs.
Viagra and Cialis continued to decline last year, beghind in generics.
Viagra's sales last year were ₩9.6 billion, down 2.3% year-on-year.
Cialis fell 1.0% from ₩6.5 billion in 2018 to ₩6.4 billion last year.
Among domestically sold erectile dysfunction treatment products, there are two products of Viagra and Cialis, and they sold a total of ₩16 billion last year.
Bayer's Levitra announced the withdrawal of the domestic market in April.
New drugs and generic products released by domestic companies account for a total of 86.0%.
After 83.3% in 2017 and 84.8% in 2018, the market share is increasing.
Domestically developed new drug products, such as SK Chemicals' 'Mvix' and 'Mvix S', and Dong-A ST's 'Zydena', also generate annual sales of ₩6 billion.
In the domestic pharmaceutical market, generics continue to strengthen in the erectile dysfunction treatment market.
In general, it is extremely unusual for generics to exceed sales of original drugs.
That's because it's not easy for generics to surpass the reliability established by original products over time.
Recently, in Korea, new drugs whose patents of multinational pharmaceutical companies have expired are increasing.
According to UBIST, Last year, Lipitor’s outpatient prescription amount was ₩17.6 billion, an increase of 8.4%.
Since 2014, the prescription amount have risen for six consecutive years.
Sanofi's anti-thrombotic drug Plavix, last year's prescription performance was ₩88.9 billion, an increase of 17.3%.
Prescription amount for Plavix increased 28.1% in two years from ₩69.4 billion in 2017.
Original drugs, such as AstraZeneca's Crestor, Boehringer Ingelheim's Twynsta, Ezai's Aricept and Novartis' Exforge, also showed a rise in prescriptions from last year.
Due to the nature of the original drug and generic insurance cap, the focus on the original increases.
On the other hand, in the non-reimbursed drug market, where suppliers such as erectile dysfunction drugs set the price, it is analyzed that generics have increased their market share by aggressively operating at lower prices than the original drugs.
Looking at the sales of erectile dysfunction treatments by company, Chong Kun Dang is facing a challenge to Hanmi Pharm.
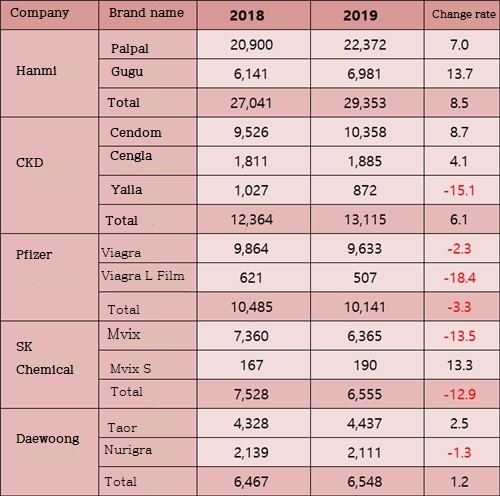
The gap was widened with latecomers with a growth rate of 8.5% year-on-year.
Chong Kun Dang recorded 6.1% year-on-year growth with the joint venture of ₩11.3 billion in three generics: Cendom, Cengla, and Yaila.
Chong Kun Dang succeeded Hanmi Pharm with a 11.5% market share.
Chong Kun Dang is gradually expanding its influence in the Viagra market even though it has fallen behind competitors.
Chong Kun Dang signed a business alliance with Bayer in 2007 and renamed Levitra under the name of Yaila, failing to enter the Viagra generic market in 2012.
Chong Kun Dang entered the Cialis market shortly after clearing his partnership with Bayer in 2015, and in 2017 launched Cengla, generic for Viagra, five years later than its competitors.
Cengla's sales last year stood at ₩1.9 billion, ranking third among the generics for Viagra after Palpal and Nurigra.
In 2018, Chong Kun Dang was licensed under Yaila, which previously sold Levitra.
Chong Kun Dang is the only company that has all three generic drugs for erectile dysfunction.
Pfizer made a joint venture between Viagra and Viagra L film last year at ₩10.1 billion.
Chong Kun Dang was the second-largest market share, down 3.3% from the previous year.
Sales of two types of erectile dysfunction treatment drugs, SK Chemicals and Daewoong Pharmaceuticals, exceeded Cialis, and ranked 4th and 5th, respectively.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









