- LOGIN
- MemberShip
- 2025-12-27 10:20:47
- Industry fears sales ban as NDMA risk in metformin rekindled
- by Chon, Seung-Hyun | translator Byun Kyung A | 2020-03-06 06:06:01

The pharmaceutical industry’s concern deepens as the same research firm that warned about ranitidine risk last year published the metformin analytic data.
Already pharmaceutical companies fear global health authorities would order a complete sales ban on metformin after they collect and probe the products.
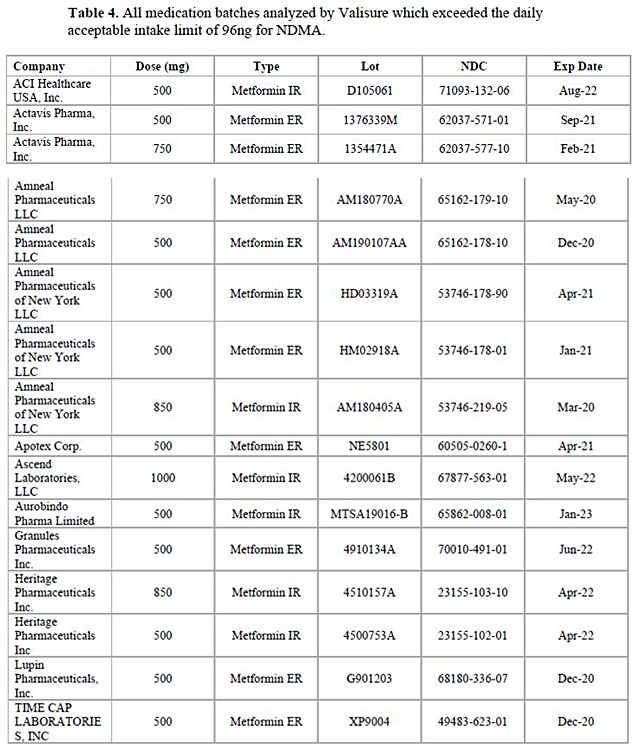
◆ Valisure “16 out of 38 metformin batches showed NDMA exceeding acceptable level” According to industry sources on Mar.
3, Valisure, a private research institute based in the U.S., has detectd cancerous N-nitrosodimenthylamine (NDMA) exceeding acceptable level of daily intake in some metformin products, which used in the U.S.
as antidiabetic drugs.
Valisure investigated NDMA in 38 metformin production batches manufactured by 22 pharmaceutical companies, following the liquid chromatography-mass spectrometry (LC-MS) protocol.
Apparently, Valisure has found NDMA exceeding acceptable level of daily intake in 16 batches from 11 companies.
One of the batches had NDMA content level 16.5 times higher than the acceptable daily intake level of 96 ng.
Valisure has requested the U.S.
Food and Drug Administration (FDA) to recall the metformin products containing levels of NDMA in excess of the daily acceptable intake limit.
On Feb.
3, the U.S.
health regulator informed their analysis on 10 metformin products distributed in the U.S.
market found two products with low level of NDMA content, which the regulator recommended not to use.
◆ Industry tenses up as Valisure warned of risk of ranitidine and nizatidine last year The pharmaceutical industry is keenly watching the private research firm, because Valisure first warned of impurity found in ranitidine last year.

and regularly publishes analytic findings regarding pharmaceutical safety.
Interestingly, Valisure’s metformin impurity risk warning is similar to the actions taken for ranitidine impurity risk.
FDA issued a statement last year announcing the presence of low levels of NDMA in ranitidine drugs like Zantac, but did not mention a specific plan to recall the product.
Since then, Valisure disclosed their own investigation showing high levels of NDMA in ranitidine drugs including Zantac, and requested FDA to recall the products.
As a result, majority of ranitidine drugs were detected with unacceptable level of NDMA and the drugs were recalled in the U.S.
and Europe.
Valisure also urged FDA to initiate investigation on nizatidine by presenting findings of NDMA in the drug.
Korea’s Ministry of Food and Drug Safety (MFDS) also decided to ban sales and recall 13 nizatidine products in last November due to the presence of excessive level of NDMA.
Such development of the decisions by global health regulators is the reason the pharmaceutical companies are tensed up about MFDS’ actions following the metformin analysis.
The risk of NDMA in metformin was first raised from Singapore.
On Dec.
4 last year, Singapore’s Health Sciences Authority (HSA) recalled three items out of 46 metformin-containing drugs they have tested.
The result confirmed high levels of NDMA exceeding the acceptable daily intake level.
MFDS is currently probing NDMA in metformin active ingredient and finished products.
Although it has already been three months since the Singaporean health regulator spoke of the risk in metformin, the Korean ministry has not yet revealed the investigation outcome.
In last December, MFDS had pharmaceutical companies to submit record of using metformin.
And on Jan.
15, the ministry presented NDMA testing methodology for metformin.
The ministry is still probing NDMA contamination in collected active metformin ingredients.
Over a month has passed since FDA has presented the interim result of metformin-NDMA investigation, but the Korean health regulator has not even mentioned of a next step.
Three days after the news of Zantac detected with NDMA in the U.S., MFDS announced interim test result of NDMA level in Zantac on last Sept.
16 and said the impurity has not been detected.
But for the ranitidine issue, the Korean government ordered sales ban on the product in a mere of 10 days.
MFDS official stated, “Currently an inspection on contamination in metformin is ongoing.” The ministry is reviewing NDMA contamination in over 900 metformin samples.
◆ Pharmaceutical companies anxious of stringent actions like ranitidine or valsartan risk Clearly, the pharmaceutical industry is edgy on the MFDS-confirmed level of NDMA in metformin from Korea and their response, because Korean regulator’s action on NDMA-detected drug so far has been more stringent than that of the U.S.
or European regulators.
MFDS has banned sales of finished product that used the controversial active ingredient at least once since January of 2015.
Many of the products were probably banned from the market even if the controversial active ingredient was not used recently.
In the U.S., only the products using active ingredient from Zhejiang Huahai Pharmaceutical were recalled by batches.
An order of complete sales ban on the product was never given.
The level of action taken by European health regulator was on par.
While every ranitidine product was banned in Korea, pharmaceutical companies voluntarily recalled the products by batches in the U.S.
and Europe.
Whereas 13 nizatidine products were banned from sales in Korea, Japan only recalled a number of the products and the U.S.
and Europe have not given a recall order.
The Korean regulator has decide to recall specific batches of nizatidine with high levels of NDMA, but those products were temporarily banned from sales until the recall was completed.
Valisure pointed out the level of NDMA in metformin varies depending on each batch, although they were manufactured by a same company.
They argued the pharmaceutical substance could be the unstable factor.
The MFDS’ decision to weed out ranitidine products was made because ‘ranitidine has an unstable quality, therefore, it is always exposed to the risk of generating NDMA.’ A tremendous chaos in the whole of pharmaceutical industry would be inevitable if the ministry is to take actions as stringent as before when it finds high levels of NDMA in some of metformin.
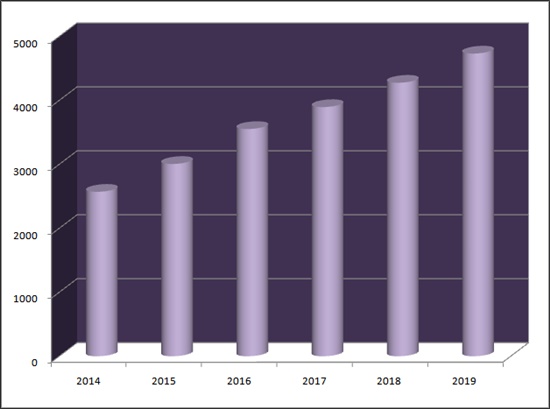
According to pharmaceutical market research firm UBIST, pharmaceuticals with metformin generated 473.2 billion won last year from outpatient prescription.
The market continues to grow as the volume has doubled since 2014.
or Europe, even if they find high levels of NDMA in some of metformin available in Korea.
Especially because a research was published claiming NDMA found in valsartan and ranitidine would not be harmful to human body.
In last December, MFDS stated, “Reviewing individual dose and administration period of patients actually taking finished product containing valsartan manufactured from Huahai Pharmaceutical with NDMA, the possibility of causing cancer was low enough to ignore.” FDA also issued a statement last November, “The risk of causing cancer by NDMA detected in ranitidine is close to the risk exposed from having barbecued meat or smoked meat.” A pharmaceutical company insider urged, “Regardless of unconfirmed harm of pharmaceuticals detected with NDMA, the produces were banned from sales and recalled.
Accordingly, pharmaceutical companies had to endure immense loss and the public’s apprehension has deepened.
On products with impurities found in the future, the Korean government should order a batch-basis recall like the U.S.
and European government have.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









