- LOGIN
- MemberShip
- 2025-12-27 08:43:42
- Lipitor tops outpatient market again amid COVID-19
- by Chon, Seung-Hyun | translator Byun Kyung A | 2020-04-21 06:27:57
The 2019 novel coronavirus infection (COVID-19) seems to not have affected the top outpatient prescription drug rank in Korea.
Dyslipidemia treatment Lipitor remained on the top of the leader board with a big gap.
The prescription volumes of anticancer Tagrisso and cholesterol-lowering combination drug Rosuzet have also continued to skyrocket.
But off-patent drugs that grew significantly in volume last year, actually showed an underwhelming growth.
According to pharmaceutical industry research firm UBIST on Apr.
20, Pfizer’s dyslipidemia treatment Lipitor has made 47 billion won in the first quarter of the year, topping the outpatient prescription volume rank.
Although the volume was 0.2 percent lower than last year’s first quarter, it easily defended the top place with almost double the volume of runner-up Tagrisso and Gliatamine.
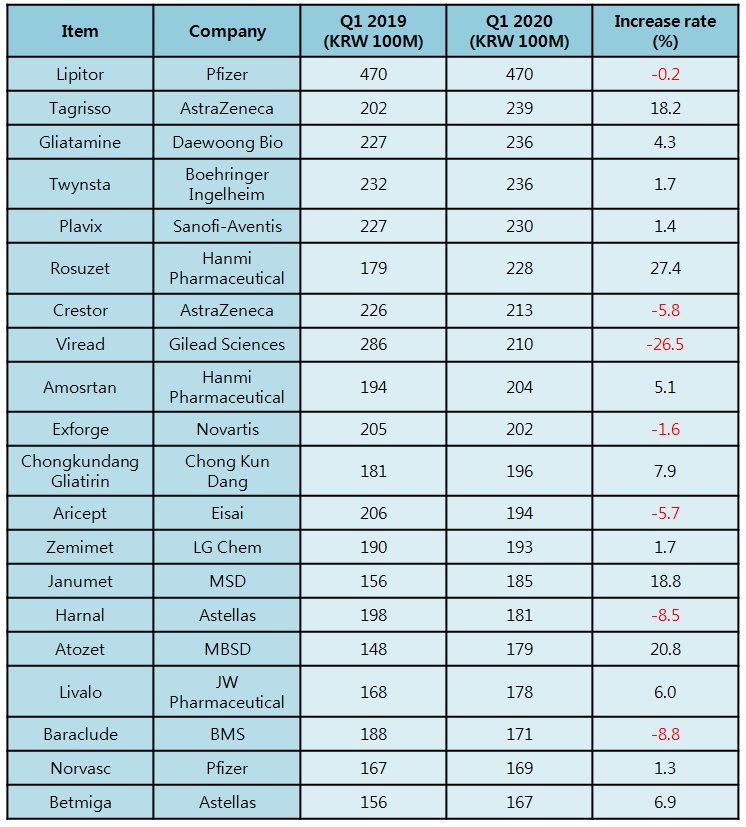
AstraZeneca’s anticancer treatment Tagrisso shined through in the market and ranked itself on the second place with 23.9 billion won in the quarter, surging by 18.2 percent from the previous year.
It was exceptional for an anticancer treatment, mostly used for inpatient, to be ranked so close to the top.
Overcoming the drug resistance issue, the drug has been labeled as a third-generation.
Tagrisso’s prescription volume has been surging since it was listed for National Health Insurance (NHI) reimbursement in December 2017.
The treatment’s outstanding effect, compared to other alternative options, and administrative convenience have seemingly accelerated the prescription volume growth.
A rosuvastatin plus ezetimibe dyslipidemia-treating combination drug, Rosuzet was released to the market in late 2015.
After acquiring the rights over ezetimibe from the patentee MSD, Hanmi Pharmaceutical was able to enter the market faster than other competitors and it has been predominantly leading the same-substance market ever since.
The combination drug has made 81 billion won from prescription last year.
Taking the rapid growth of Rosuzet into account, the sources even project the drug could be the first Korean-made drug to surpass annual prescription volume of 100 billion won.
LG Chem’s combination agent diabetes drug Zemimet has made 15.6 billion won in the first quarter last year, and the volume was increased by 18.8 percent this year at 18.5 billion won.
Zemimet has combined Zemiglo, a new diabetic drug with DPP-4 inhibitor solely developed by LG Chem, and metformin.
A combination agent for dyslipidemia Atozet showed a 20.8-percent growth in prescription over a year and generated 17.9 billion won in the first quarter.
Released by MSD in 2015, Atozet is an atorvastatin plus ezetimibe combination drug.
Currently, Chong Kun Dang holds the co-sales deal signed by the multinational company.
In the first quarter, off-patent drugs have shown stagnant growths.
Boehringer Ingelheim’s hypertension treatment Twynsta has generated 23.6 billion won and made only 1.7-percent growth from the year before.
The prescription volume of Sanofi’s anticoagulant drug Plavix grew 1.7 percent from the year before and marked 23.6 billion won in the quarter.
Compared to last year, AstraZeneca’s dyslipidemia treatment Crestor and Gilead’s Viread have recorded 5.8 percent and 26.5 percent decrease in prescription volumes, respectively.
The prescription volumes of Eisai’s Alzheimer’s disease treatment Aricept and of Astellas’ benign prostatic hyperplasia treatment Harnal-D also had each dropped by 5.7 percent and 8.5 percent.
Except for Viread, these off-patented originals had a significant surge in prescription last year.
In 2019, Lipitor has grown 8.4 percent from the year before and generated 176.2 billion won.
Plavix’ prescription volume reached 88.9 billion won last year with 17.3 percent surge from the year before.
Some original drugs like Crestor and Arisept marked a 10-percent growth in prescription volume last year, when Harnal-D also grew by 6.5 percent.
However, the research firm analyzed the originals’ prescription volumes have diminished this year as the generics have performed better.
The industry views that the continuous spread of COVID-19 has influenced the outpatient prescription market.
When the multinational pharmaceutical companies preemptively stopped visiting healthcare institutes due to the outbreak, the Korean pharmaceutical companies could have persuaded the prescribers to switch to generics.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









