- LOGIN
- MemberShip
- 2025-12-21 13:02:44
- Pharma closely watches tariffs as U.S. sales surge
- by Chon, Seung-Hyun | translator Hong, Ji Yeon | 2025-05-09 06:00:29
South Korea’s pharmaceutical and biotech companies are focusing on potential tariffs on U.S.
exports of their medicines.
President Donald Trump’s announcement that tariffs would be imposed on imported pharmaceuticals has left major exporters considering devising countermeasures.
Celltrion, whose North American sales have topped KRW 1 trillion, has pre-distributed over a year’s inventory.
GC Biopharma, which recently entered the U.S.
plasma product market, hopes to secure tariff exemptions on U.S.-sourced raw materials.
Companies such as Samsung Biologics, SK Biopharmaceuticals, and Daewoong Pharmaceutical have been accelerating their penetration of the U.S.
market, making it imperative to monitor the ramifications of any tariffs closely.
Celltrion completes transfer of 15 months’ inventory to prepare for tariffs… last year’s North American sales surpassed KRW 1T On the 8th, industry sources reported that Celltrion posted a statement on its website on the 7th titled 'Our Position and Response Strategy Regarding the Trump Administration's Tariff Policy in the U.S.,' announcing that it 'has completed the transfer of approximately 15 months' worth of inventory for products scheduled for sale in the United States in 2025.' Celltrion explained that it has minimized the impact of tariffs on this year's U.S.
sales and projected sales in the first half of next year.
Celltrion stated, "We have finalized a manufacturing agreement for finished products with a local U.S.
CMO, and have already secured the volume that can be produced domestically.
We have also completed measures to negotiate additional contracts with manufacturing sites to cover any further volumes that may be affected by tariffs." President Trump's declaration on the 5th that "within the next two weeks, we will announce item-by-item tariffs on pharmaceuticals" prompted Celltrion to unveil these detailed countermeasures.
President Trump also signed an executive order directing regulatory agencies to shorten approval times for pharmaceutical plants built in the United States.
Since President Trump took office, Celltrion has introduced three separate countermeasures to address potential tariffs on U.S.
exports, demonstrating an active approach to contingency planning.
On January 30, Celltrion announced measures stating, "We have secured sufficient inventory for our products currently sold in the U.S.
to be sourced locally without additional imports through at least the third quarter of 2025." In February, the company announced the completion of a nine-month inventory transfer.
It stated, "We have finalized measures to minimize the impact in 2025," once again presenting the status of its tariff mitigation plans alongside further inventory transfers.
Celltrion said, "We have already developed strategies to minimize tariff impacts beyond next year," adding, "Regarding the establishment of local API manufacturing facilities in the U.S.
as part of our long-term response, we have completed preliminary reviews and are now conducting a comprehensive, detailed assessment."
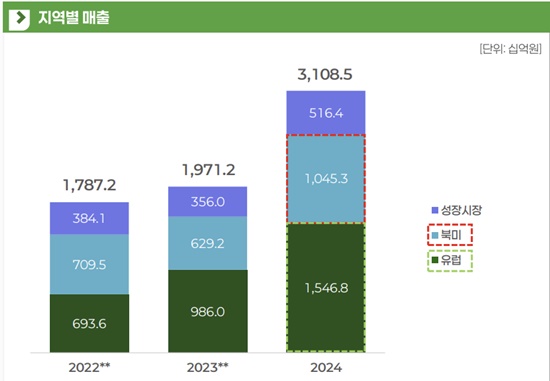
Celltrion has achieved U.S.
approval for a total of 11 pharmaceutical products.
Celltrion's first U.S.
approval came in 2016 with Inflectra, the biosimilar to Remicade.
In 2018, its biosimilars to the oncology drugs MabThera and Herceptin received FDA approval.
In September 2022, Celltrion obtained FDA marketing authorization for Vegzelma, its Avastin biosimilar, and in 2023, its Humira biosimilar Yuflyma received FDA approval.
In August 2023, the FDA approved Celltrion's subcutaneous (SC) formulation of Remsima, marketed as Zymfentra, as a standalone product.
Last December, its Stelara biosimilar Steqeyma was approved by the FDA.
This year, Celltrion has secured U.S.
approval for four biosimilars.
In January, the FDA approved Aptozma, its Actemra biosimilar for treating autoimmune diseases.
In March, it received FDA approvals for Stobocloo, its biosimilar to bone disease treatments Prolia and Xgeva, and Omlyclo, its Zolair biosimilar, also cleared the U.S.
regulatory hurdle.
Last year, Celltrion's biopharmaceutical sales in the North American market reached KRW 1.0453 trillion.
While its North American sales fell from KRW 709.5 billion in 2022 to KRW 629.2 billion in 2023, they surged by 66.1% last year, surpassing KRW 1 trillion for the first time.
GC Biopharma begins U.S.
sales of plasma product Alyglo… forecasts that "tariffs on U.S.-sourced materials will have limited impact" GC Biopharma, which recently entered the U.S.
plasma product market, has anticipated limited tariff impacts.
In December 2023, GC Biopharma entered the U.S.
market when the FDA approved its plasma product, Alyglo.
Alyglo is a liquid immunoglobulin preparation purified from plasma fractions.
It is used to treat primary immunodeficiency disorders such as congenital immunodeficiency and immune thrombocytopenia.
In its Q1 earnings release, GC Biopharma stated that, because a tariff exemption for U.S.-sourced raw materials is being considered, it expects the impact of tariffs to be limited.

pharmaceuticals will not significantly affect the plasma product Alyglo (source: GC Biopharma) The raw material of blood plasma for Alyglo is produced in the United States.
GC Biopharma acquired 100% of ABO Holdings for KRW 138 billion in December last year.
ABO Holdings is based in California and operates six blood banks across New Jersey, Utah, and California.
GC Biopharma produces Alyglo at its Ochang facility using blood supplied by ABO Holdings, and then sells it in the U.S.
GC Biopharma said, "We have secured inventory of Alyglo in the U.S.
and are reviewing CMO partnerships for finished product manufacturing," outlining its tariff strategy.
In Q1, plasma product sales were KRW 127.2 billion, up 42.3% from KRW 89.4 billion during the same period last year.
GC Biopharma began full-scale sales after shipping the initial Alyglo batch in July last year.
In Q2 2023, plasma product sales were KRW 90.6 billion, rising 50.8% to KRW 136.6 billion in Q3 and increasing to KRW 161.7 billion in Q4.
While plasma product sales fell by 21.3% in Q1 2024 compared to Q4 2023, they remained above KRW 100 billion for three consecutive quarters.
Green Cross sells Alyglo in the U.S.
through its subsidiary, GC Biopharma USA, which recorded its first sales of KRW 48.6 billion last year.
U.S.
Sales Growth for Samsung Biologics, SK Biopharm, Daewoong surges… Monitoring Tariff Impacts Closely Korean pharmaceutical and biotech firms must closely watch for tariff impacts as their U.S.
sales have recently increased.
According to the Korea Customs Service, pharmaceutical exports to the U.S.
reached USD 1.35809 billion (approx.
KRW 1.61 trillion) last year, up 50% year on year.
The U.S.
accounted for 18% of total pharmaceutical exports.
Exports to the U.S.
have risen rapidly over the past two years: from KRW 843.94 billion in 2022 to USD 903.30 million (approx.
KRW 1.07 trillion) in 2023, a 7% rise, and last year’s surge accelerated further.
In 2022, the U.S.
overtook Germany as South Korea’s largest pharmaceutical export market, maintaining that position for three consecutive years.
Recently, orders for contract development and manufacturing (CDMO) have expanded, and sales of new drugs and biosimilars in the U.S.
have increased, driving a significant rise in export volumes.
Of Samsung Biologics’ KRW 45.473 trillion in sales last year, KRW 11.741 trillion (25.8%) came from the U.S.
Samsung Biologics’ U.S.
revenue share was 28.5% in 2022 and 26.3% in 2023.
Samsung Biologics calculates regional sales based on the location of its CDMO clients.
Samsung Bioepis, Samsung Biologics’ biosimilar subsidiary, is also expected to be affected by U.S.
tariffs.
Of its KRW 1.5277 trillion in sales last year, KRW 917.5 billion (60.1%) came from Europe and KRW 610.2 billion (39.9%) from non-European regions.
Within those non-European sales, the U.S.
represents the largest share, and they grew by 46.6% year on year.
Since 2017, Samsung Bioepis has obtained U.S.
approval for ten biosimilars.
Starting with its Remicade biosimilar in 2017, followed by Herceptin, Enbrel, and Humira biosimilars in 2019.
Ontruzant in September 2019, Eticovo, and Hadlima in April and July 2019, respectively; the Lucentis biosimilar Byooviz in September 2021.
Since last year, Samsung Bioepis obtained FDA approval for its biosimilars in five areas, including biosimilars of Eylea, Stelara, Soliris, Prolia, and Xgeva.
Samsung Bioepis’ biosimilar supply chain involves API production at Fujifilm Bio’s Danish plant and Samsung Biologics, with finished products made by overseas CMO partners and sold worldwide.
Its U.S.
biosimilar sales are handled by Biogen, Organon, Teva, and other partners.
SK Biopharm’s new epilepsy drug Xcopri is also accelerating its U.S.
penetration.
FDA-approved in November 2019, Xcopri has been sold directly in the U.S.
via SK Life Sciences since May 2020.
SK Biotech produces the Xcopri API, and a Canadian CMO partner supplies the finished drug.
Last year, Xcopri’s U.S.
sales reached KRW 438.7 billion, up 62.1% year on year, with cumulative U.S.
sales of KRW 969.5 billion.
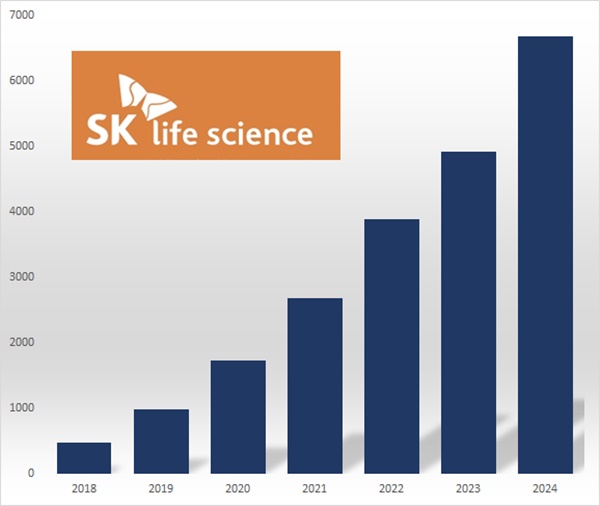
Over two years, its sales grew 72.0%, from KRW 388.3 billion in 2022, more than fourteen-fold over six years since KRW 47.5 billion in 2018.
SK Group has established U.S.
manufacturing through SK pharmteco, which oversees CMO operations.
SK pharmteco runs five companies, including SK Biotech, SK Biotech Ireland, Ampac, Yposkesi, and CBM.
Ampac has facilities in California, Texas, and Virginia.
Among traditional pharmaceutical companies, Daewoong Pharmaceutical is the most active U.S.
exporter, selling its botulinum toxin product Nabota (FDA-approved via partner Evolus in 2019) from its Hyangnam plant.
Nabota’s exports were KRW 49.2 billion in 2021, more than doubling to KRW 109.9 billion in 2022, then rising to KRW 117.4 billion and KRW 156.0 billion in 2022 and 2023, respectively, over triple in three years.
Q1 2024 exports were KRW 37.3 billion, four times the domestic sales of KRW 8.3 billion.
U.S.
subsidiaries of Korean pharma firms also saw strong growth.
Hugel America, a subsidiary of Hugel, is responsible for the U.S.
sales and R&D of the botulinum toxin product “Retivo.” Its revenue increased by 86% year-over-year, from KRW 21.1 billion in 2023 to KRW 39.2 billion last year, and its net loss of KRW 19.2 billion turned into a net profit of KRW 15.5 billion.
Huons USA, operated by Huons, markets products such as lidocaine injections.
Its revenue rose 29%, from KRW 1.1 billion to KRW 1.4 billion, and its net loss of KRW 1.7 billion in 2023 turned into a net profit of KRW 0.1 billion last year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










