- LOGIN
- MemberShip
- 2025-12-27 06:57:02
- COVID-19 affected off-patent drug market share plunge?
- by Chon, Seung-Hyun | translator Byun Kyung A | 2020-05-11 06:18:42
Although off-patent originals have been consistently expanded their market shares in recent years unaffected by the army of generics entering the market, the original’s market share shrunk in last first quarter.
The Korean pharmaceutical industry experts analyze the originals struggled to grow in the market against intense marketing of generics amid COVID-19 pandemic.
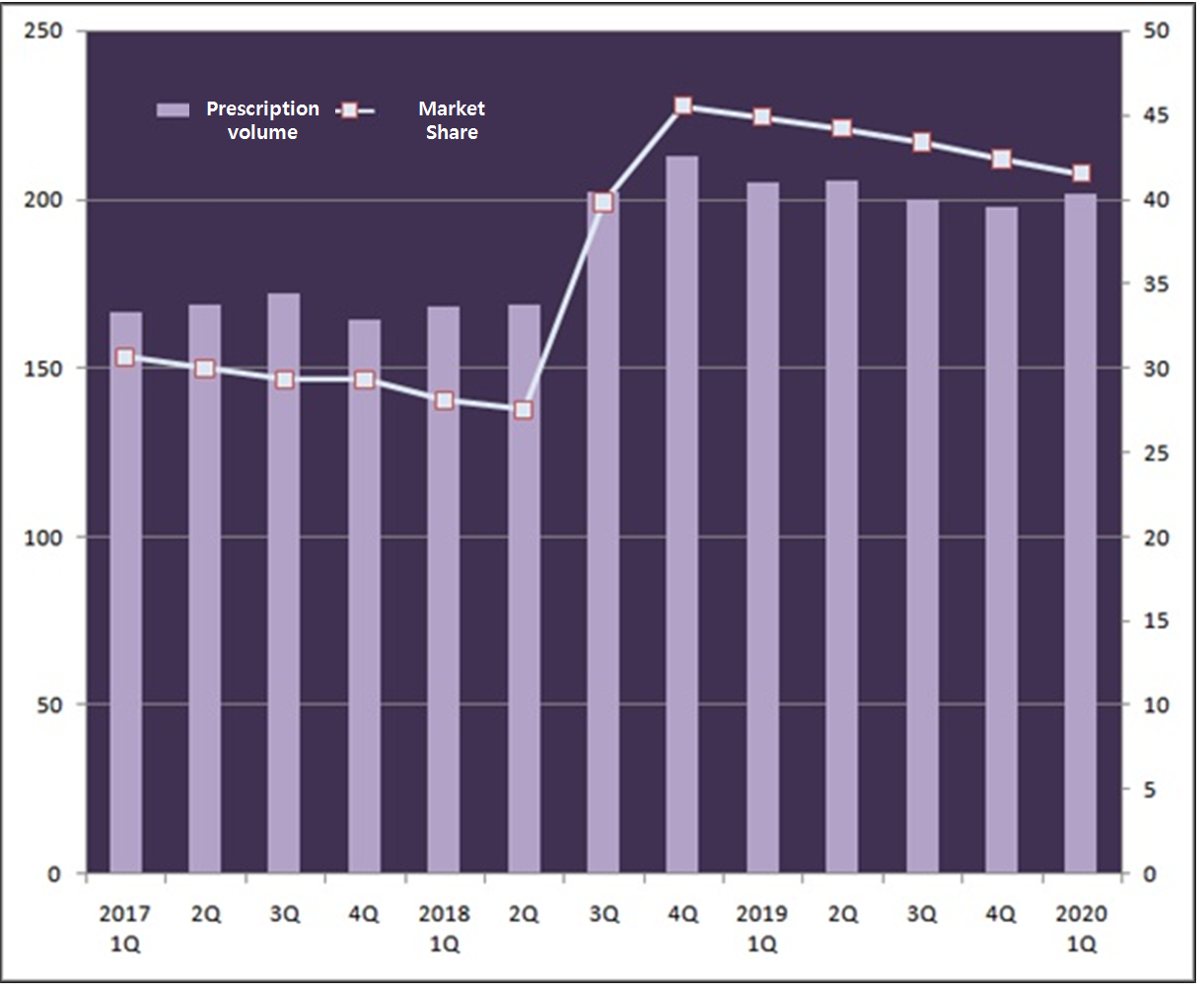
◆Lipitor and Crestor market shares stagger in the first quarter On May 10, pharmaceutical industry research firm UBIST stated Pfizer’s antihypertensive Lipitor (atorvastatin) has generated 47 billion won from outpatient prescription in the first quarter showing 0.2 percent decrease from the same time last year.
Its prescription volume has been surging for last few years, but the growth seems to have slowed down.
Compared to the first quarter last year, the prescription volume has increased only by 5.5 percent.
The overall prescription volume of individual atorvastatin drugs in the first quarter reached 138.2 billion won with 2.3 percent increase from the year before.
The overall market volume has grown but the original’s prescription volume has gone down.

Even compared to the fourth quarter (34.92 percent) last year, the first quarter’s volume fell by 1 percent point.
Since the third quarter of 2016 (33.66 percent), Lipitor’s quarterly market share has hit the lowest point in 42 months.
Regardless of over 100 generics entering the market from 2009 as Lipitor’s patent was expired, the original has been constantly growing in the market.
Lipitor’s market share steeply expanded from 31.70 percent in the first quarter 2015 to 34.92 percent in the fourth quarter last year.
Although it maintained the market share over 34 percent since the fourth quarter of 2016, the figure plummeted this year at around 33 percent.
Besides Lipitor, many of off-patent drugs that showed off their strong growth until last year saw their market share drop, simultaneously.
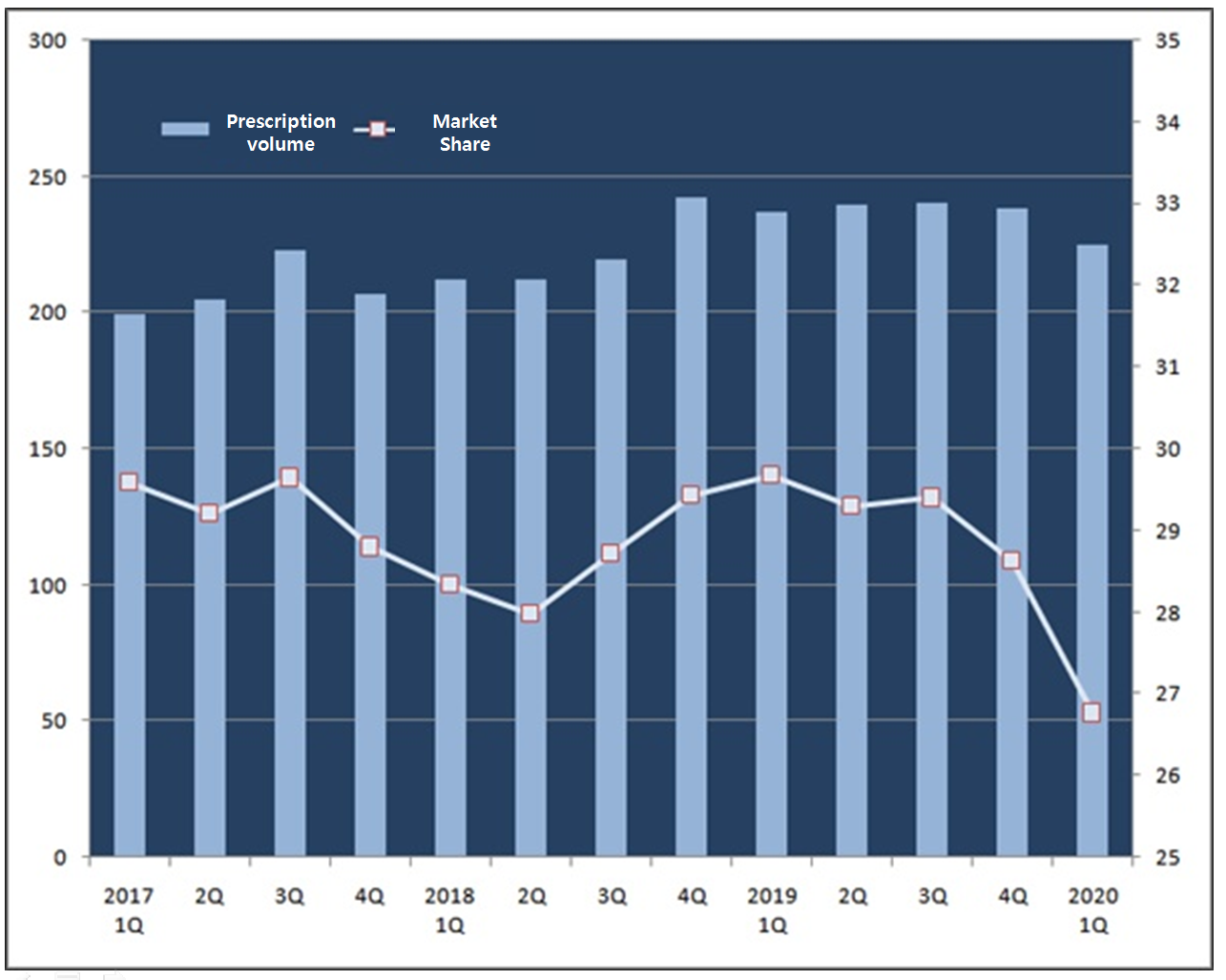
Compared to the previous year, Crestor had an 11.8-percent growth in last year’s first quarter, generating 23.7 billion won.
The overall individual rosuvastatin drug market in the past first quarter has generated 84.1 billion won, jumping 5.2 percent from the same time last year.
Despite the general growth in the market, Crestor’s first quarter market share has gone down significantly from 29.67 percent last year to 26.76 percent this year.
Even against the last fourth quarter, this year’s first quarter market share fell by 2 percent point from 28.63 percent.
Although it defended the market share of around 30 percent against over 100 generics for a while, this year’s first quarter market share has hit the lowest point.
◆Prescription volumes in Plavix, Aricept and Exforge also plunge The anticoagulant agent clopidogrel’s market also demonstrated similar trend.
The first quarter prescription volume in Plavix (clopidogrel) was at 23.0 billion won with 1.4 percent increase than the previous year.
The growing tendency continued but it was sluggish.
In last year’s first quarter, Plavix generated 22.7 billion won, increased by 10.7 percent from the year before.
In the first quarter of 2018, the drug’s prescription volume was 6.8 percent higher than the previous year.
The overall individual clopidogrel drug market generated 2.6 percent more in the first quarter this year at 89.8 billion won, compared to 87.6 billion won in 2019.
In the first quarter clopidogrel market, Plavix’ share was brought down to 25.65 percent by 0.38 percent point from 25.93 percent in last year.
Compared to the fourth quarter last year at 26.27 percent, this year’s first quarter share was decreased by 0.62 percent point.
The drug’s quarterly market share recorded the lowest in 18 months since the third quarter of 2018.
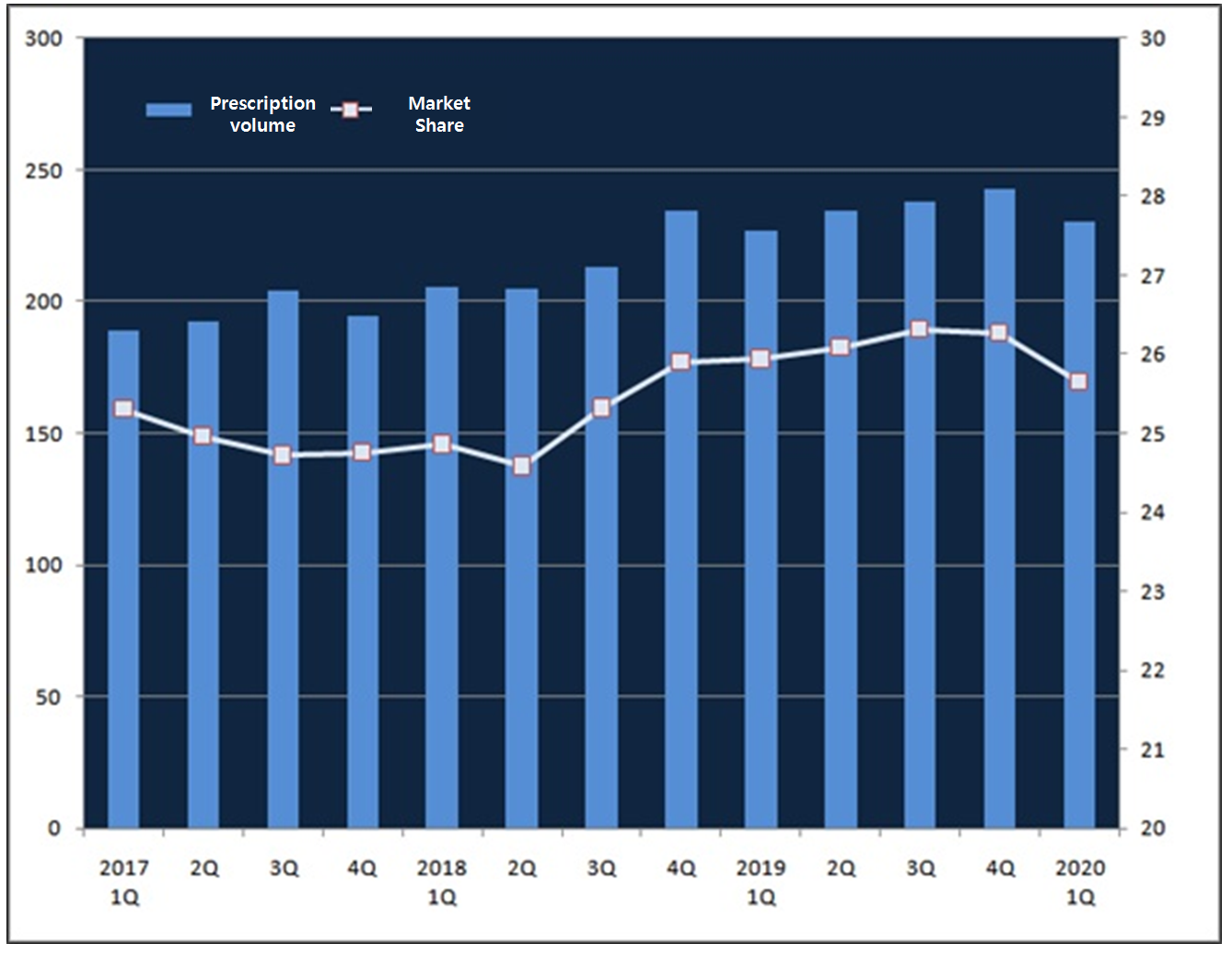
The overall market of an Alzheimer’s disease-associated symptom treating agent donepezil has made 58.2 billion won this past first quarter with 1.0 percent increase from the same time the year before.
Compared to 21.6 billion won made last year, the original brand drug Aricept has only made 23.0 billion won in last first quarter, recording 6.0 percent fall.
In 2017 and 2018, Aricept’s first quarter prescription volume marked 18.9 billion won and 20.9 billion won, respectively, and showed a continuous growth but it turned downward this year.
Aricept’s market share in the first quarter within the donepezil market has hit the lowest at 37.21 percent.
Compared to last year at 39.99 percent, the first quarter share this year fell by 3 percent point.
And from the fourth quarter last year, the number was again dropped by 0.3 percent point.
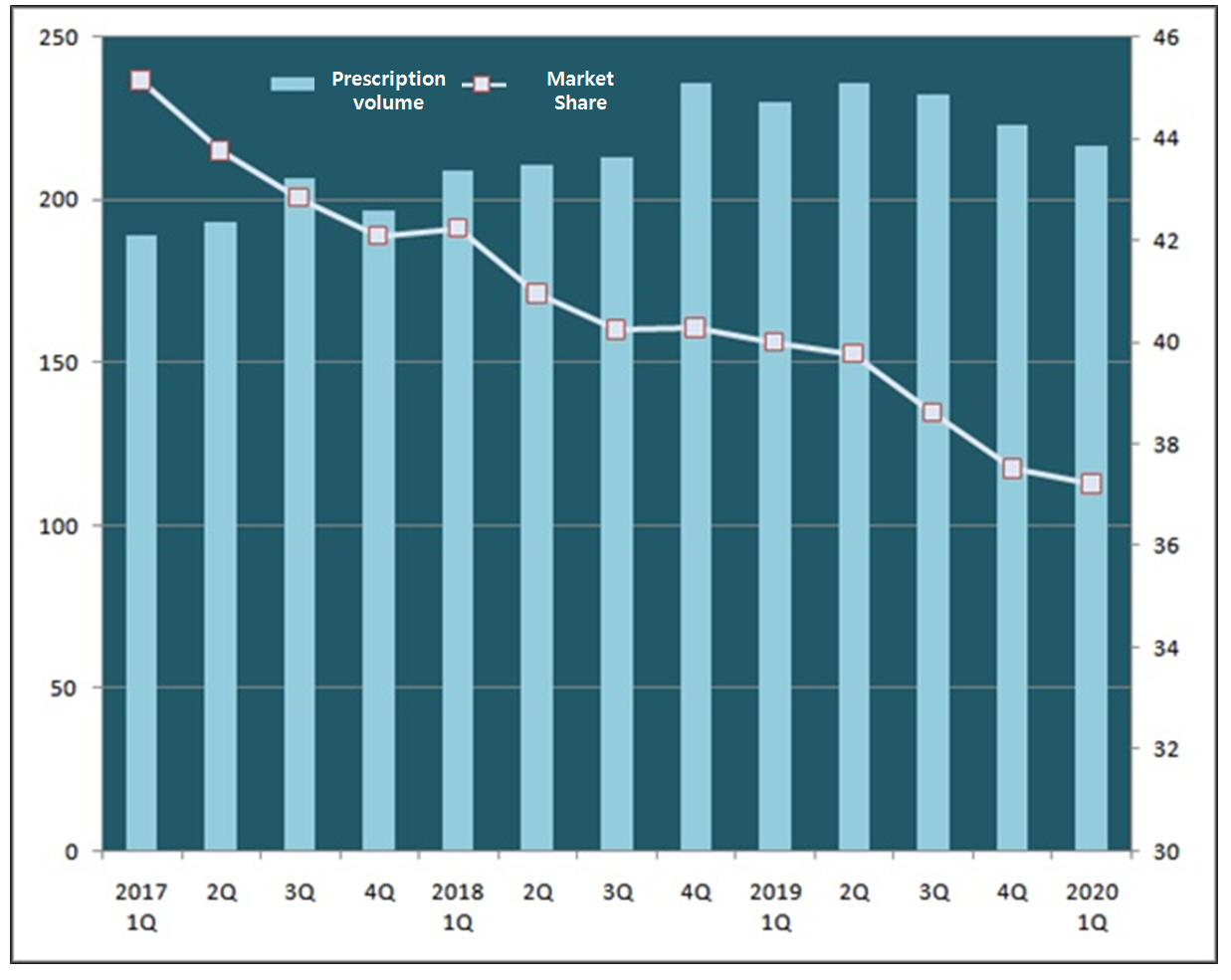
The first quarter prescription volume was at 20.2 billion won marking 1.6 percent drop from the year before.
In the first quarter of last year, Exforge has generated 22.0 percent more in prescription volume against the year before.
Influenced by the impurity found in valsartan in July 2018, the prescription volume of Exforge has surged but it has been stagnating recently.
Taking up 41.54 percent of the combination drug market in this year’s first quarter, Exforge’s share has plummeted by over 3 percent point from 44.91 percent last year.
The industry experts claim the originals have advantages in market share growth due to Korea’s unique drug pricing system.
When a generic is first released to the market, the original’s reimbursed price is reduced down to 70 percent of its initial pricing.
And after a year from then, the pricing is again adjusted to 53.55 percent of the pricing before the patent expiration.
The maximum pricing of a generic can be up to 59 percent of the original’s price before the patent expiration, and a year later the price also drops to 53.55 percent of the original’s pre-patent expiration price.
The experts argue the prescribers seemingly prefer originals as it gets priced on par with generic pricing before the patent expiration.
In this year, however, generics’ market share has gone up significantly.
In this first quarter, the overall generic outpatient prescription volume has reached 3.70 trillion won with a 2.7-percent surge from last year at 3.60 trillion won.
The industry experts analyze most of the original market has been engulfed by generics.
When multinational pharmaceutical companies have halted their salespeople from visiting healthcare institute amid COVID-19 outbreak, Korean companies could have assertively approached the healthcare providers and convinced them to prescribe generics.
Since the first confirmed case of COVID-19 in Korea on Jan.
20, a growing number of pharmaceutical companies have refrained from visiting healthcare institutes, starting from the multinational companies.
Most of the pharmaceutical company salespeople have started working from home as the number of confirmed cases skyrocketed since the discovery of the 31st confirmed case on Feb.
19.
The experts also speculate many of chronic disease patients could have received prescription for a longer period of time at once to avoid visiting their healthcare providers.
They also noted more clear trend in prescription drug market would be visible after April or May, when the pandemic heavily influenced the prescription volume.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









