- LOGIN
- MemberShip
- 2025-12-27 03:50:24
- ↓generic's approval as the drug price system is implemented
- by Chon, Seung-Hyun | translator Choi HeeYoung | 2020-08-26 06:22:43
The number of approval for generic drugs has declined for the second consecutive month.
If the bioequivalence test is not performed, the new drug price system, which gives low drug prices, was implemented, and greatly reduced the number of new licenses for authorized generics.
Some point out that the government's move to strengthen generic regulations has caused an unprecedented confusion as pharmaceutical companies have already installed as many generics as possible.
According to the MFDS on the 13th, a total of 70 generic prescription drugs were approved last month.
Following 73 in June, it fell short of 100 for the second consecutive month.
The number of generic licenses recorded more than 100 for 17 consecutive months from January last year to May this year.
During this period, an average of 323 new generics per month were granted.
In May of last year, 560 generics were licensed in a month, and more than 200 new generics were launched every month this year.
In May, 412 generics were licensed, but from June the number of licenses dropped significantly.
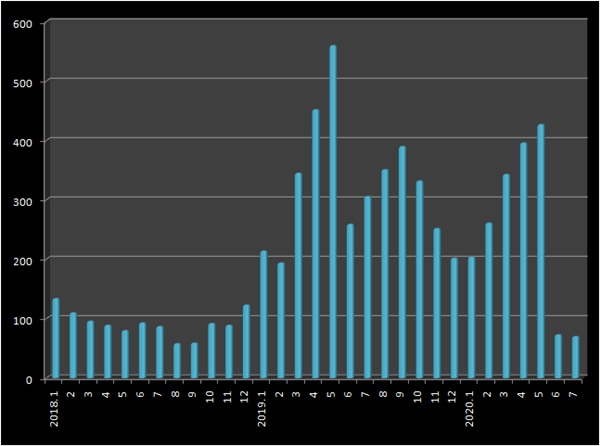
This is because it tried to get as many generics as possible before the regulation was strengthened.
.The new drug price system, which was implemented from this month, is pointed out as a factor in the surge in generic licenses
.Generic products must meet both the direct bioequivalence test and the use of the registered drug substance to maintain the 53.55% upper limit price compared to the original drug before the current patent expiration
.Whenever one requirement is not met, the upper limit goes down by 15%
.If one of the two requirements is satisfied, the upper limit is lowered to 45.53%, and if there is no satisfaction requirement, the upper limit is lowered to 38.69%
.The reorganized drug price system includes a stepped drug price system in which the upper limit is lowered as the time for registration of benefits is delayed
.If more than 20 generics are listed in the specific ingredient market, the upper limit of the newly listed item will be up to 85% of the existing lowest price
.It is pointed out that the Ministry of Food and Drug Safety's move to tighten regulations on permits has also encouraged the approval of a bunch of generics
.In April of last year, the MFDS announced a reinforcement of regulations on joint bioequivalence test through a partial amendment to the “Regulation on Pharmaceuticals Approval, Notification and Review”
.This is the content of limiting the number of consigned manufacturers to one original manufacturer
.Although the reinforcement of joint bioequivalence test regulations was not initiated due to the recent recommendation of the Regulatory Reform Committee of the The Office for Government Policy Coordination, the number of applications for generic licenses increased significantly shortly after the plans to strengthen bioequivalence test regulations were revealed earlier last year
.According to the MOHW, the previous drug price system is applied to products applied for benefit registration in May
.Generics, which have been approved since June and applied for reimbursed registration, are said to receive low drug prices by applying the new drug price system
.Even if both the bioequivalence test and the requirements for the registered drug substance are satisfied, the price of the drug is further reduced as the stepped drug price system is applied
.This is the background of the sharp decline in the number of new generic licenses from June
.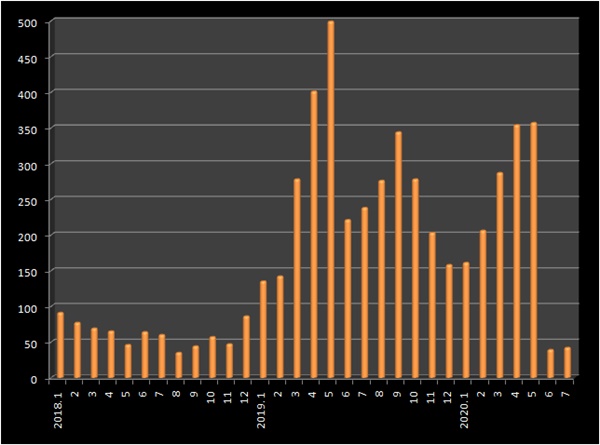
.The number of commissioned generics licensed in June and July was 39 and 42, respectively, which was less than 20% of the average of 273 from January to May this year
.Last year, a total of 3173 new generics were entered
.This means that an average of 264 commissioned generics per month poured out
.The proportion of authorized generics to the total number of generic licenses is also on a decline
.Among the new licensed generics in June and July, the proportion of authorized generics was 53% and 60%, respectively
.From January to May, authorized generics accounted for 84% of licensed generics
.Last year, the proportion of authorized generics reached 82%
.It is interpreted that the number of attempts to enter new markets decreased as authorized generics could no longer receive high drug prices due to the implementation of the new drug price system
.As pharmaceutical companies have been intensively licensed for generics within a short period of time, the number of areas for further entry has greatly decreased
.Most of the generics that pharmaceutical companies could sell before the tightening of regulations were granted
.The number of generics licensed for last year is more than five times that of 741 in 2018
.Recently, pharmaceutical companies have tried to apply the previous drug price system for the last time
.From January to May this year, around 300 drugs were newly listed on the reimbursed list each month
.Following 558 drugs in June and 653 in July, 836 products were newly added to the list in August
.That's about 2,000 in three months
.It is an unprecedented generic listing in the government's regulatory reinforcement movement in the market.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









