- LOGIN
- MemberShip
- 2025-12-27 02:14:12
- Obesity drug market expands despite COVID-19 as Qsymia soars
- by An, Kyung-Jin | translator Byun Kyung A | 2020-08-28 06:17:34

The treatment market expanded, although the consumers became reluctant to open their wallets amid COVID-19 pandemic.
A newcomer Qsymia is now leading the market alongside with Saxenda, or so-called ‘Gangnam Diet Shot.’ On Aug.
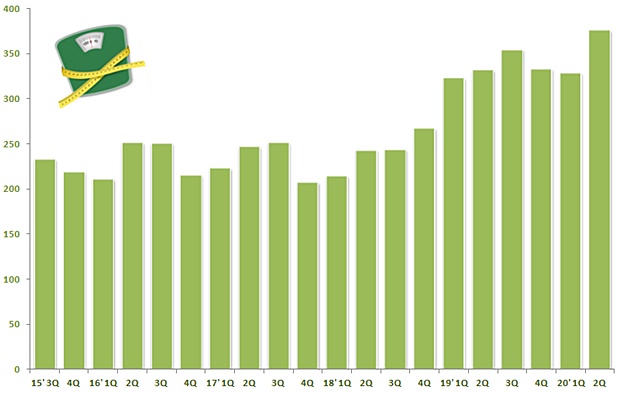
26, a pharmaceutical market research firm IQVIA found Korea’s obesity treatment market in the second quarter jumped 13.2 percent from last year at 33.2 billion won to 37.6 billion won.
The cumulative sales in the first half of the year grew 7.6 percent from last year at 65.4 billion won and generated 70.4 billion won.
If the market continues to see the positive growth, it would be able to break through the 100 billion won mark for two consecutive years.
In the first half of the year alone, Saxenda made 18.3 billion won.
Compared to last year at 19.8 billion won, the quarterly sales dropped by 7.4 percent, but the injection is still the top obesity treatment in the Korean market.
Its market share in the first half of the year was at 26.0 percent.
Saxenda was the world’s first obesity treatment to be approved as a glucagon-like peptide-1 (GLP-1) receptor agonist.
Saxenda shares the same substance liraglutide with Victoza, prescribed to type 2 diabetes patients, but its administration route and dose are different.
As the users became aware of the medication to be comparatively safer with its mechanism of working like appetite-regulating and weight loss-inducing GLP-1, Saxenda has been enjoying unprecedented popularity for last two years.
Started off with 2018 third quarter sales at 1.7 billion won, Saxenda’s sales surged to 5.6 billion won in the fourth quarter and took the lead in the market.
Ever since the sales in first quarter of 2019 broke through 10 billion won point, Saxenda has maintained the sales around 10 billion won and defended the market leader title.
In the third quarter last year, the treatment sales peaked and it dominated market share of 33.7 percent.
But Saxenda’s easy race was shaken up, when Qsymia has announced its release in last January.
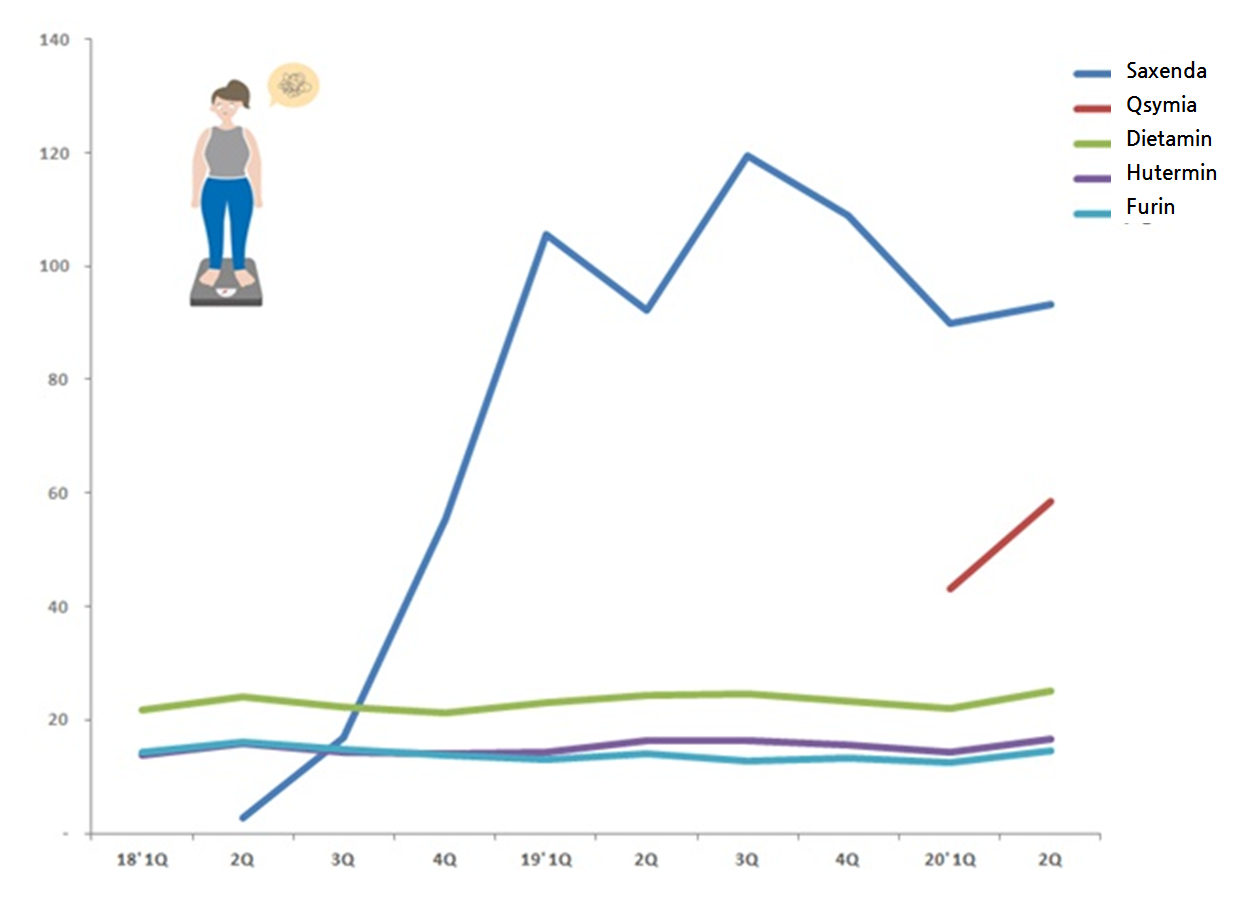
After making another 5.8 billion won in the second quarter, Qsymia accumulated 10.2 billion won in the first half of the year and consolidated its strong market presence next to Saxenda.
As of the first half of 2020, Qsymia’s market share has reached 14.4 percent.
The figure doubles the market share of Daewoong Pharmaceutical’s Dietamin (6.7 percet) that used to be the second in the market until the fourth quarter last year.
Alvogen Korea licensed in phentermine and topiramate combined Qsymia from the U.S.-based pharmaceutical company Vivus in 2017 for the sales in the South Korean market.
Alvogen Korea also signed a co-marketing deal with Chong Kun Dang late last year and came into the market with powerful sales and marketing activities.
Well experienced in the obesity treatment market with Furing and Furimin, Alvogen Korea created a mutual synergy effect with Chong Kun Dang’s vast sales power and quickly took over the market.
Except for Qsymia, other treatment generally experienced stagnating growth.
Three products out of the top five—Daewoong Pharmaceutical’s Dietamin, Huon’s Hutermin and Alvogen Korea’s Furing—saw their sales fall compared to last year same time.
Hutermin made 3.1 billion won and raised the sales only by 0.8 percent from last year, whereas Dietamin (4.7 billion won) and Furing’s (2.7 billion won) sales volume fell by 0.2 percent and 0.4 percent, respectively, against last year.
The market was expecting an intense competition eyeing on the market share left by a blockbuster drug Belviq as it exited the market with risk of developing cancer.
But the market experts say the pandemic and release of Qsymia have grounded them from soaring in the market.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









