- LOGIN
- MemberShip
- 2025-12-27 00:23:57
- Court to rule against companies on eye drop pricing?
- by Chon, Seung-Hyun | translator Byun Kyung A | 2020-09-07 06:12:37
The end in two years of legal dispute over the eye drop pricing reduction seems to be approaching.
The Supreme Court has ultimately ruled against pharmaceutical companies on one of the two pricing reduction litigation cases.
And as the government has won both the first and second trials over the other case, it would be unlikely for the pharmaceutical company to win the last appeal.
◆MOHW authorizes pricing reduction on eye drops twice, the Supreme Court’s final decision made on one The pharmaceutical industry sources reported on Sept.
6, South Korea’s Ministry of Health and Welfare (MOHW) has announced the execution suspension on the pricing reduction in 33 eye drops would be lifted.
The ministry’s announcement followed the Supreme Court’s decision.

3, the Supreme Court dismissed the appeal made by pharmaceutical companies regarding the government’s attempt to bring down the pricing on eye drop products.
The Court stated, “The appellant’s claim for the appeal is clearly unjustifiable, therefore, all appeals would be dismissed.” In December 2018, MOHW has decided to lower the pricing on 33 eye drop products.
As the affected pharmaceutical companies filed litigation against the decision, they also requested the suspension of execution on the ministry’s action.
As the court accepted the request, the pricing reduction was withheld, but the pricing was brought down, nonetheless, with the Supreme Court’s final decision.
The legal dispute between the government and pharmaceutical companies over the pricing reduction in eye drops lasting over two years is getting closer to the end.
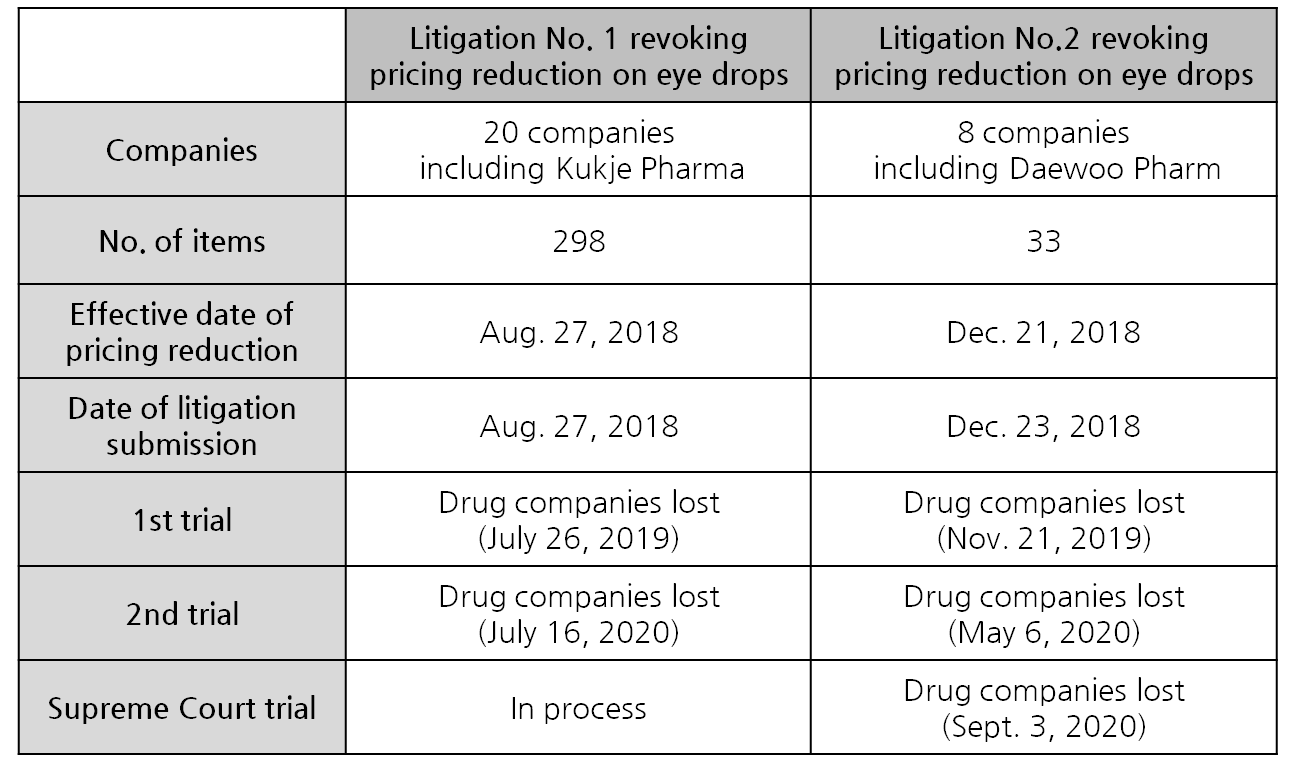
Two separate litigations were conducted, both aiming to revoke the pricing reduction in eye drop products.
On Aug.
27, 2018, the Ministry revised its list of reimbursed drugs and upper limit pricing to bring down the pricing of 307 eye drop items by up to 55 percent.
The key change was to apply the same pricing standard on single-use eye drops with same concentration of pharmaceutical substance (dose per mL), regardless of the overall amount of the solution.
Regarding the ministry’s decision, 20 pharmaceutical companies claimed the pricing reduction on 299 items is unfair, and filed litigation to the Seoul Administrative Court to nullify the government order.
Kukje Pharma, Daewoo Pharm, Daewoong Bio, DHP Korea, BINEX, Samchundang Pharm, Sinsin Pharm, CMG Pharma, Youngil Pharm, Inist Bio Pharmaceutical, Ildong Pharmaceutical, Chong Kun Dang, Taejoon Pharmaceutical, Korea Global Pharm, Hanlim Pharm, Hanmi Pharmaceutical, Humedix, Huons and Huons Medicare were part of the litigation.
The Seoul Administrative Court ruled in favor of the government in July last year.
The group of pharmaceutical companies filed for an appeal, and the Seoul High Court came to the same decision in last July.
As the companies have pushed case on, the Supreme Court is currently reviewing the case.
Four months after MOHW’s decision to reduce the eye drop pricing, the ministry has again unveiled a partially revised list of reimbursed drugs and upper limit pricing that lowered pricing on 33 eye drop items by eight companies on Dec.
21, 2018.
The pricing reduction was ordered on eye drop items listed after the first pricing reduction order, and the ministry meant to apply the same standard.
The litigation was filed by Daewoo Pharm, Sinsin Pharm, Youngil Pharm, Reyon Pharmaceutical, Ildong Pharmaceutical, Hanlim Pharm, Huons and Huons Medicare.
The eight companies filed the litigation to revoke the ministry’s order to lower the pricing.
In November last year, the Seoul Administrative Court has ruled in favor of the government body, and the court reaffirmed its earlier denial of the litigation during the second trial held in last May.
Although the order was given later than the first one, the case was processed faster and reached the Supreme Court’s decision earlier.
Accordingly, the 20 pharmaceutical companies await the Supreme Court’s ruling on the first pricing reduction order.
Nevertheless, as the courts have ruled against the companies in two prior trials, the Supreme Court would be unlikely to rule otherwise.
The pharmaceutical companies argued the pricing reduction is an unfair order as the ministry has abused the discretionary power, violated the principle of trust protection, and taken illegitimate proceedings, but the courts denied the claim.
And the Supreme Court has discontinued the trial and dismissed the case.
The discontinuance of the trial refers to the Supreme Court dismissing the case without a trial, when the court sees no difference in viewing the prior rulings.
The Supreme Court basically made a final decision without even holding the trial.
◆Confusion in drug pricing as the court dismissed and reaccepted the request to suspend the pricing reduction Confusion in the pricing in the affected eye drops was caused as the court once dismissed and accepted the request to suspend the execution of pricing reduction order again.
While the pharmaceutical companies filed two litigation cases to revoke the pricing reduction orders, they also requested the court to order suspension of execution.
And only one request to withhold the pricing reduction order was accepted.
Prior to the first trial with 20 pharmaceutical companies, the companies submitted an application to request the Seoul Administrative Court to suspend MOHW’s pricing reduction on eye drop until 30 days after the court’s decision.
Eventually, the pricings were lowered, as of Sept.
22, 2018, when the Seoul Administrative Court dismissed the request.
But the companies, again, requested the suspension to the Seoul High Court, which accepted the request and recovered the eye drop products’ original pricing, as of Nov.
30, 2018.
Every time the litigation cases proceeded to the higher court, the companies requested for the court to suspend the execution, and the rest of the requests were all accepted.
Currently, the ministry’s order to lower pricing on 20 companies’ 287 items, related to the first litigation, is withheld.
Although the number of items started from 299, the number has gone down due to some companies withdrawing the litigation or reimbursement.
The Supreme Court would soon make the decision on the rest of 287 items.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









