- LOGIN
- MemberShip
- 2025-12-27 00:23:52
- The contradiction of generic exclusivity
- by Kim, Jin-Gu | translator Choi HeeYoung | 2020-09-07 06:13:43

This is the case when the rule for '1st place' in a marathon is set to be '14 seconds after the first goal scored'.
Competition will become meaningless, and only fights will increase.
The achievements of legitimate efforts that the first place should receive will be shared by numerous 'joint first prizes'.
The controversy over the generic exclusivity system is similar.
Due to the regulation that suppresses discrimination, the 'contradiction of common first place' is prevalent.
It is often observed that up to 45 items receive the right at the same time, and the product is not released even after receiving the right.
As the circumstances look like this, the profits from obtaining generic exclusivity are very small.
It is calculated that each item is limited to ₩400 million for 9 months.
This is the reason why criticism is constantly raised for promoting free rides over the current system.
It is also the reason why the pharmaceutical industry is regretting about the government's revised right.
The purpose of the introduction was to provide benefits for the efforts of active pharmaceutical companies in the development of generics and patent challenges.
However, as of five years later, most pharmaceutical companies with the generic exclusivity do not benefit.
Dailypharm surveyed the prescriptions of products with generic exclusivity since 2015.
The average prescription amount for 9 months during the sales period was only about ₩400 million.
According to the MFDS, as of the end of August 2020, 140 generics were launched on the market within the generic exclusivity period.
The prescription performance of these items during the period was ₩59.3 billion.
It is calculated that the average prescription amount per item (₩59.3 billion ÷ 140 items) is only ₩420 million for 9 months.
Although there is a difference in the size of the market and the loyalty of the original for each item, criticism has been raised that it is not effective given the nature of the generic exclusivity.
This is why pharmaceutical companies, which have led the patent challenge, are complaining that it is 'free ride'.
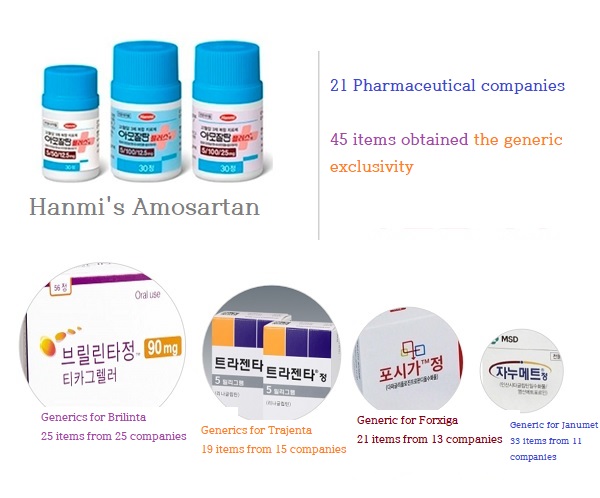
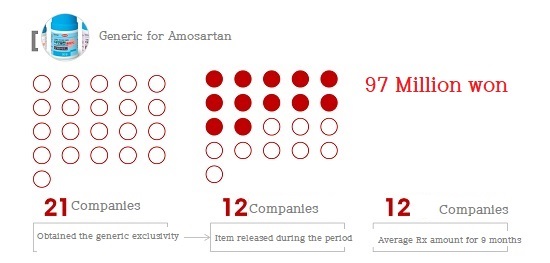
In the case of generics for Amosartan (Amlodipine+Losartan), 21 companies received the right of the generic exclusivity with 45 items.
Of these, only 12 (57%) launched products during the period.
12 pharmaceutical companies only produced a total of ₩1,160 million during the period (May 9, 2015 to April 1, 2016).
This is less than ₩100 million per pharmaceutical company.
If the rights were monopolized by one pharmaceutical company rather than 21 pharmaceutical companies, it is expected that the results would not have reached ₩100 million.
The rest of the products are similar.
In the case of generics for Layla, out of the 14 companies that received the right, only 10 launched the product within the period, and they only recorded an average of ₩430 million during this period.
13 companies for generics for Viread received the right, but only 11 companies released the product within the period.
The company that launched the product produced an average of ₩370 million per company.
Nine companies challenged generics for Feburic, but only 8 released products, and they only achieved an average of ₩79 million won per company.
On the other hand, items with fewer competitors tended to have more profits from generic companies.
Hanmi alone challenged the patent for Patanol eye drop (Olopatadine)' and received the right of the generic exclusivity as a generic called Olotadine.
During the period, Hanmi recorded ₩1.35 billion in prescriptions.
Dong-A ST challenged the patent for Dilatrend (Carvedilol) and received the right as a generic called Vasotrol.
During the period, Dong-A ST produced a prescription record of ₩990 million.
◆Generic for Amosartan, In addition, the mess of the generic exclusivity is observed in many products.
As of the end of August 2020, there are 387 items that have acquired the right of copyright.
Excluding items with different capacity in the same lineup, it is calculated that 244 products have received the right.
There are 42 original drug patents that these 244 products have overcome.
It means that one original product has an average of 5.8 generics and received the right.
This trend is observed to be more frequent in larger items.
In the case of Amosartan, 21 pharmaceutical companies received the right for 45 items.
It means that only 21 people are tied for first place.
Other products are also very similar.
Most recently, on the 25th of last month, 13 pharmaceutical companies received rights with 21 items on Forxiga (Dapagliflozin).
In 2016, Januvia (Sitagliptin) acquired 22 items from 10 companies, and Janumet (Sitagliptin + Metformin) acquired 33 items of 11 companies.
14 companies and 10 companies, respectively, succeeded in acquiring the right for natural drugs, Stillen 2X and Layla.
◆Even after winning the right, half of them were not released within the period.
Why? Although many pharmaceutical companies flocked to acquire the right of the generic exclusivity, it is also confirmed that not many pharmaceutical companies actually launched the product.
As of the end of August of this year, there are a total of 255 items that have reached the right sales period or are currently in use, of which only 140 items have actually released products to the market within the right sales period.
Only 5 out of 10 products (54.9%) that received the right copyright have put products on the market.
There are many reasons for not releasing a product even after obtaining the right through patent disputes.
The reason is that it won the right, but failed to prove bioequivalence for the release of generics, felt a burden as the patent dispute with the original company passed to the second or third trial, or judged that it was not marketable.
However, it is easy to obtain the right of the generic exclusivity as the number of requests for trial increases.
◆Initial request for trial '14 days' It was introduced in 2015.
With the conclusion of the Korea-US FTA, the licensed patent linkage system was introduced in 2012, and the system that grants monopoly rights to patent challenge generics through step-by-step enforcement procedures was also revealed.
All three requirements must be met in order to obtain the right of copyright.
First, it is necessary to first request a trial (confirmation of invalid or passive scope of rights) for the original patent.
Second, it must win the patent trial raised in this way.
Third, it is necessary to first apply for approval for generics.
Acquiring the right seems tricky because all three conditions must be completed, but it is not.
This is because the clues are attached to the first requirement, the request for an initial trial.
Under the current law, it stipulates that 'company that makes a claim within 14 days after the initial request for a judgment is also recognized'.
This regulation causes the contradiction of the joint first place.
After winning the referee, the day after the end of the PMS (reexamination), a bunch of applications for permission continued.
It was common for dozens of companies to be bound by consignment and got the right at the same time.
The first request for judgment became a ticket for the sale of generics.
It is not known exactly why the '14 days' rule in question was included when making the law.
Some say that this is because the time it took to be published in the patent gazette after a request for a trial was filed for about 14 days.
However, it is said that Korea is the only case in the world that regulates 14 days.
A patent expert in the pharmaceutical industry said, "The generic exclusivity in Korea has been reduced to a system that has little practical benefit.
Due to the scattered initial request for trial, only pharmaceutical companies that challenged patents have been changed to a structure that is relatively damaged."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









