- LOGIN
- MemberShip
- 2025-12-27 00:23:54
- Botulinum share in domestic market is 93%
- by Chon, Seung-Hyun | translator Choi HeeYoung | 2020-09-09 05:47:12
Among domestically produced and imported botulinum toxin preparations, products developed by Korean companies accounted for more than 90%.
Following Medytox, Hugel, and Daewoong Pharmaceutical, Huons Global and Pharma Research joined.
The three types of Meditoxin, whose license was decided to be canceled, recorded ₩82.2 billion in production last year.
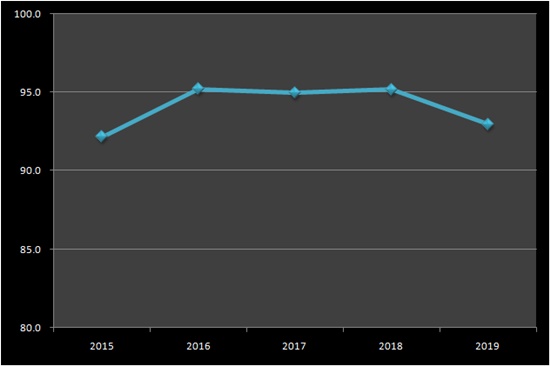
According to the MFDS on the 7th, the domestically produced and imported botulinum toxin formulation last year was ₩213.5 billion.
It decreased by 0.3% from ₩214.2 billion in 2018, but increased 2.5 times in 4 years from ₩85.5 billion in 2015.
This is the result of a survey of products that reported production and import performance to the MFDS.
For imported products, an exchange rate of ₩1,200 per dollar was applied.
The botulinum toxin formulations registered last year's production and import records are a total of 10 products including Meditoxin, Botulax, Nabota, Innotox, Botox, Xeomin, Liztox, Rientox, Coretox, and Dysport.
A domestic company, Medytox, has three products, including Meditoxin, Innotex, and Coretox, and the remaining companies are selling one product each.

The production of botulinum toxin preparations of domestic companies such as Meditoxin, Botulax, Nabota, Innotox, Liztox, Rientox, and Coretox last year was ₩198.5 billion, accounting for 92.2% of the total.
The proportion of domestically developed botulinum toxin production decreased slightly from 95.2% in 2018, but considering that Allergan and Ipsen entered generic maraket, the share of over 90% is an unusually high proportion.
Some of the products produced by domestic companies are sold in overseas markets, but even if exports are excluded, they dominate the original imported products.
Production performance of Meditoxin was ₩104.1 billion last year, accounting for nearly half of the total production and imports, but decreased by 13.9% from ₩120.8 billion in 2018.
However, Innotox and Coretox, generics for Meditoxin, are recording high growth with production values of ₩10.5 billion and ₩2.5 billion respectively last year.
In the case of Meditoxin, the license was canceled in June due to the suspicion of manipulating documents, except for 200 units out of the four doses of 50, 100, 150, and 200 units.
Last year's production of the three products that were canceled last year amounted to ₩82.2 billion, or 78.9% of the production of Meditoxin.
While the court cited an application for suspension of execution of the three types of Meditoxin's license cancellation, collection, and disposal orders last month, it became possible to sell even the main lawsuit.
If sales are finally banned, it is expected that a significant loss in sales will be inevitable for Medytox.
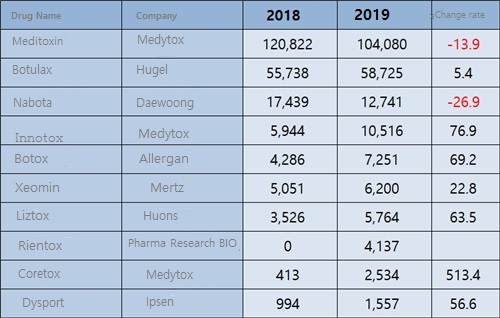
Huons Global recorded ₩5.8 billion in production of Liztox, a botulinum toxin drug developed last year.
After recording production of ₩2.3 billion for the first time in 2016, it has increased every year, including ₩3.2 billion in 2017 and ₩3.5 billion in 2018.
Huons Global received a license for export under the brand name Hutox in October 2016, and then received an official license under the product name Liztox in April 2018.
Phamar Reserch BIO’s “Rientox” recorded the first production performance of ₩4.1 billion last year.
Phamar Reserch BIO is a company that changed its name after BioC&D, a bio company, was acquired by Phamar Reserch Product in January 2018.
It obtained a permit for export in February of last year, and although it has not yet been released in Korea, it seems that sales have occurred in overseas markets.
Allergan's Botox imports last year stood at ₩7.3 billion, up 69.2% from the previous year, but it was far short of domestically developed products.
The report of Xeomin by Mertz and Dysport by Ipsen also posted only ₩6.2 billion and ₩1.6 billion last year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









