- LOGIN
- MemberShip
- 2025-12-27 00:23:55
- Hanmi to turn efpeglenatide crisis around with new partner
- by An, Kyung-Jin | translator Byun Kyung A | 2020-09-10 06:24:31

Four months after when the global company indicated its intention to return the rights on the drug May, Sanofi has decided to suspend clinical studies on the antidiabetic treatment and officially informed each clinical institutes.
Hanmi Pharmaceutical plans to take over all five Phase III clinical trials Sanofi was conducting, and look for new opportunities to commercialize efpeglenatide.
Over the past three years, three global Phase III clinical trials on efpeglenatide have recruited 5,391 participants, and collected a vast amount of data.
Various options are in discussion, such as finding a new commercialization partner in the antidiabetic treatment field, exploring new indications treating other metabolic diseases, and developing a combination therapy with the Korean company’s new drug pipeline.
◆Sanofi says “R&D strategy has changed,” and returns the rights over efpeglenatide after five years Apparently, the sign of the contract termination was looming from last year.
CEO Paul Hudson at Sanofi, held an investors event to talk about R&D strategies in late last year.
He declared the company "would halt researches in the diabetes and cardiovascular programs." His plan is to focus R&D investment in four areas, including oncology, blood disorders, rare disease, and neurology diseases.
Even at the time, Sanofi had unchanged commitment to continue developing the long-lasting GLP-1 agonist efpeglenatide.
The company was determined to complete the five Phase III clinical trials on efpeglenatide, but wanted find another commercialization partner to take charge of global sales and marketing activities after receiving the approval.
CEO Paul Hudson affirmed, “It was the best decision for the successfully launch of efpeglenatide while maximizing the productivity of our research engine.
It was irrelevant to efficacy and safety of the substance, and it would make no changes on license-in agreement with Hanmi Pharmaceutical." However, Sanofi overturned its initial position in just five months, and informed Hanmi Pharmaceutical of its intention to return the rights over efpeglenatide.
The two companies concluded the efpeglenatide returning procedure on Sept.
8 (local time) after a 120-day negotiation period as stated on the contract terms.
Hanmi Pharmaceutical would not take any legal actions like litigation for damage compensation the company has previously claimed.
Sanofi is expected to hand over all data collected from the on-going Phase III clinical trials to Hanmi Pharmaceutical, and step away from the entire development process.
Hanmi Pharmaceutical has also agreed to take over the one Phase III clinical trial to be completed in October.
Including the compensation and research cost coverage, the conditions for breaching the contract were undisclosed.
◆Market competition intensified with oral GLP-1 agonist Rybelsus The pharmaceutical industry pin points the cause of Sanofi giving up on developing efpeglenatide lies on the pipeline growing unattractive for the market.
The global GLP-1 agonist market is expanding rapidly.
Nevertheless, the industry sees that the latecomers would struggle to enter the market as multinational pharmaceutical companies like Novo Nordisk and Eli Lilly are firmly dominating the market.
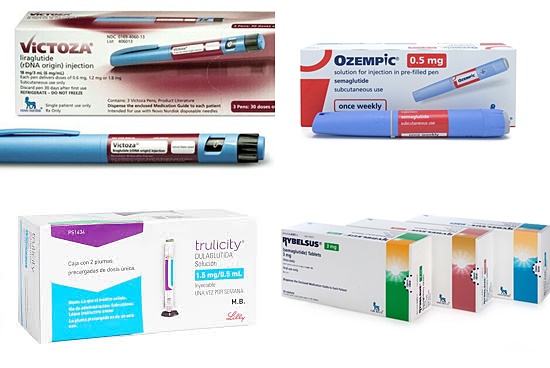
In addition, Novo Nordisk is expanding its market presence as it added newly launched the world's first oral GLP-1 agonist Rybelsus (semaglutide) to its existing line up of once-daily subcutaneous injection 'Victoza (liraglutide)' and once-weekly injection 'Ozempic (semaglutide).'.
Lily's Trulicity generated global sales of USD 4.23 billion (approximately 4.91 trillion won), surging by 29 percent compared to the previous year.
The drug is expected to reach its new record in yearly sales as it made 2.50 billion dollars in the first half of this year.
Novo Nordisk's three GLP-1 agonists have earned overall DKK 33.22 billion (approximately 6.26 trillion won).
The Danish company’s first GLP-1 Victoza had a sales dip of 21.93 billion kroner, but the company had even more significant growth with the recently launched Ozempic sales increasing up to 11.24 billion kroner, and newly released Rybelsus making 50 million kroner.
In the first six months of the year, the Ozempic sales grew up to 9.60 billion kroner, and surpassed Victoza sales reaching 9.23 billion kroner.
Rybelsus marked six-month sales of 584 million kroner immediately after its release.
The three drugs’ sales in total reached 19.4 billion kroner (approximately 3.65 trillion won) in the first half of the year.
A GLP-1 agonist efpeglenatide has extended the administration term to once-weekly by incorporating Hanmi Pharmaceutical’s key platform technology, ‘Lapscovery’.
However, the industry experts analyze the investigational drug’s competitiveness as a follow-on drug is faltering when Trulicity and Ozempic dominate the once-weekly injection market and even an oral option Rybelsus emerges.
◆Hanmi with 6,000 participants’ data, committed to rekindle efpeglenatide’s potential The industry experts address Sanofi’s three-year worth of abundant clinical data as an engine to bring efpeglenatide back to life.
Sanofi has been operating five Phase III clinical trials since it signed the deal over efpeglenatide from Hanmi Pharmaceutical in 2015.
Two years into the deal, a Phase III AMPLITUDE-M study that compared efpeglenatide against placebo on the blood glucose level reduction effect was initiated from late 2017, and four Phase III clinical studies have started as of last year.
Following are the four Phase III trials; AMPLITUDE-D study compares efpeglenatide against Trulicity plus metformin combination therapy; AMPLITUDE-O study evaluates efpeglenatide’s effects on the cardiovascular system in type 2 diabetes patients; AMPLITUDE-L study administers efpeglenatide and other existing insuling treatment to type 2 diabetes patients; and AMPLITUDE-S additionally administers efpeglenatide to type 2 diabetes patients, who cannot control blood sugar level even after using metformin alone or metformin and sulfonylurea combination.
AMPLITUDE-M, AMPLITUDE-D and AMPLITUDE-O studies have completed subject registration.
Total 5,391 participants registered for the three studies.
Considering the number of participants registered for the remaining two studies, the number would sum up to around 6,000.
Hanmi Pharmaceutical plans to conduct and complete the AMPLITUDE-M study scheduled to end in October.
AMPLITUDE-M study is the first Phase III clinical study initiated by Sanofi after it licensed in efpeglenatide.
Companied called for participants from 56 research institutes in four countries including the U.S., Germany, Poland, and the U.K.
from December 2017, and compiled the roster with 406 participants by early September last year.
The endpoint of the study is to evaluate the change of goal is to evaluate the rate of change in hemoglobin A1c at week 30 of the treatment.
The entire study is scheduled to conclude by next month after the participants’ visits and data collection.
Based on the efpeglenatide data collected so far, Hanmi Pharmaceutical is determined to turn the crisis around with a plan to seek a new commercialization partner and also by opening options to explore new indications and research about combined treatment effect with other Lapscovery-based pipelines.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









