- LOGIN
- MemberShip
- 2025-12-27 00:23:53
- Korean rare disease drug Hunterase market share surges
- by An, Kyung-Jin | translator Byun Kyung A | 2020-09-29 06:20:45

The treatment’s market share gap with Elaprase (idursulfase) that used to dominate the Hunter syndrome market has tripled as Hunterase maintains market share of over 70 percent.
While the treatment is generating significant sales in Central America and North Africa, its commercial value is to grow even further as it prepares for the Chinese and Japanese markets.
A pharmaceutical market research firm IQVIA reported on Sept.
24, GC Pharma’s Hunterase has generated 8.8 billion won in the second quarter of the year.
The sales in first half of the year accumulated to 17.6 billion won, taking up 72.3 percent of the market.
Although the figure went down by 0.5 percent compared to 17.7 billion won last year same time, the treatment has been dominating the market with the sales still tripling the competitor’s.
In the same time, Sanofi Genzyme’s Elaprase has only made 6.7 billion won.
In 2008, GC Pharma has licensed in the technology from Samsung Medical Center Professor Jin Dong Kyu and co-developed Hunterase to treat patients with Hunter syndrome.
After Ministry of Food and Drug Safety (MFDS) green lighting the drug in 2012, the Korean company successfully commercialized the world’s second Hunter syndrome treatment, following after Sanofi Genzyme’s Elaprase.
Hunter syndrome is a rare, inherited disorder, diagnosed roughly in one out of 100,000 to 150,000.
Caused by deficiency of an enzyme called idursulfase, the disease has poor prognosis as patients express skeletal abnormality, cognitive impairment other unpredictable symptoms when not treated in adequate timing and have life-expectancy of around 15 years.
There are about 70 to 80 cases reported in South Korea.
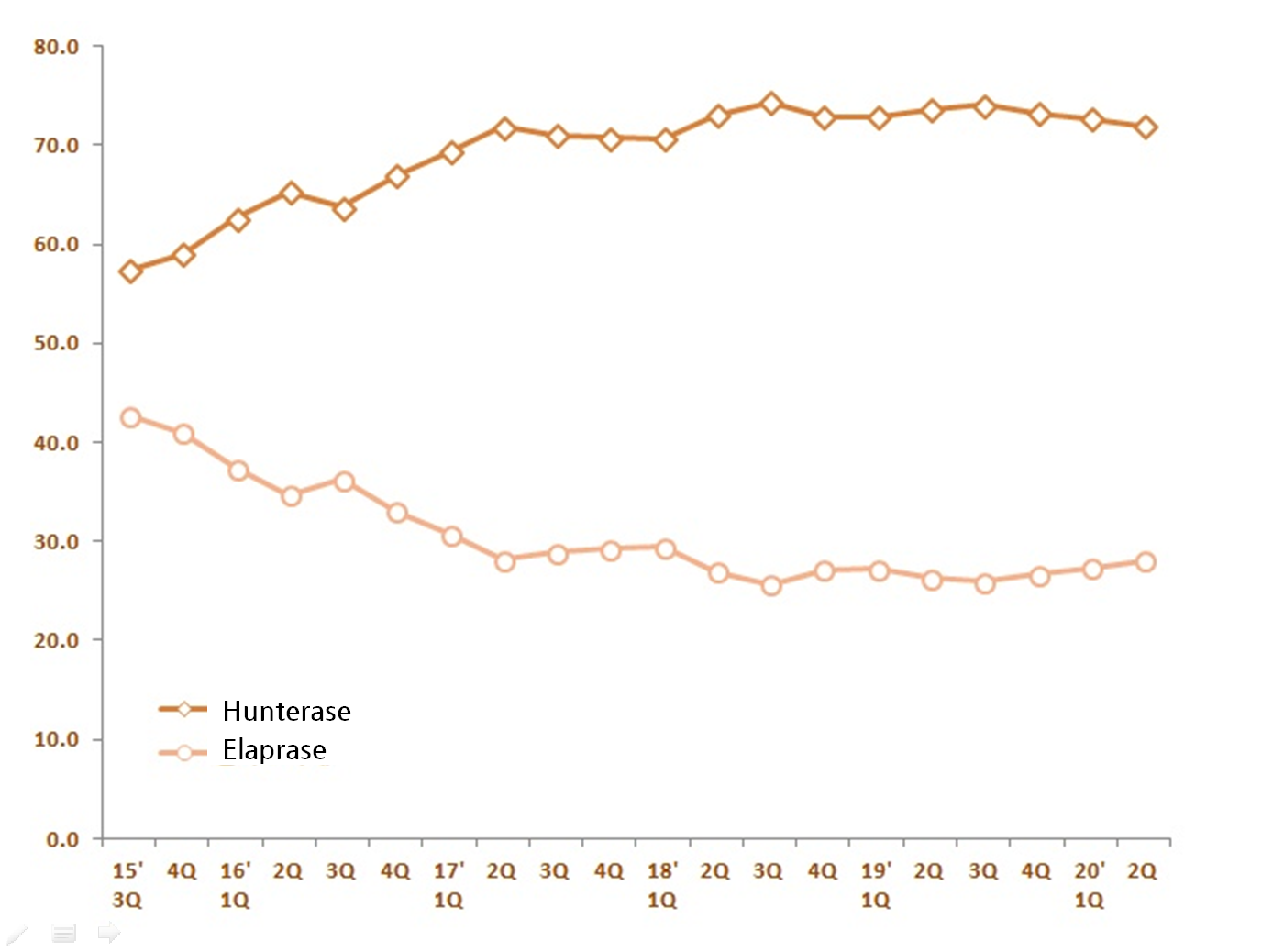
Although it used to generate 7 billion won per quarter, Elaprase’s quarterly sales have plunged to around 3 billion won ever since Hunterase entered the market in the third quarter of 2012.
The global drug has raised 3.4 billion won in the second quarter taking up the market share of 28 percent.
Basically, the two-thirds of the market were handed to Hunterase.
Hunterase’s market presence has been consistently growing since it was released.
According to IQVIA’s data, the sum of two Hunter syndrome treatments in last year’s second quarter marked 12.2 billion won, which has gone up by 17.1 percent from the second quarter of 2016.
When Elaprase’s sales were stagnating for last four years, Hunterase has been pushing the market expansion.
The Korean drug’s maximum price with coverage is 2,254,200 won, which is about 17.6 percent lower than Elaprase (2,651,616 won).
As the follow-on drug was offered with lower pricing, the Hunter syndrome treatment market has been expanded and the rare disease patients’ access to treatment was improved.
Currently, Hunterase is indicated to be administered 0.5 mg per kilogram of patient’s body weight once-weekly.
For instance, a child weighing 36 kg would need to pay 6,762,600 won to receive 18 mg per dose.
In a year, the number would add up to 351.66 million won.
Because the number of competitors is low with limited number of patients, the companies are expecting high profit for providing life-long doses of the needed enzyme.
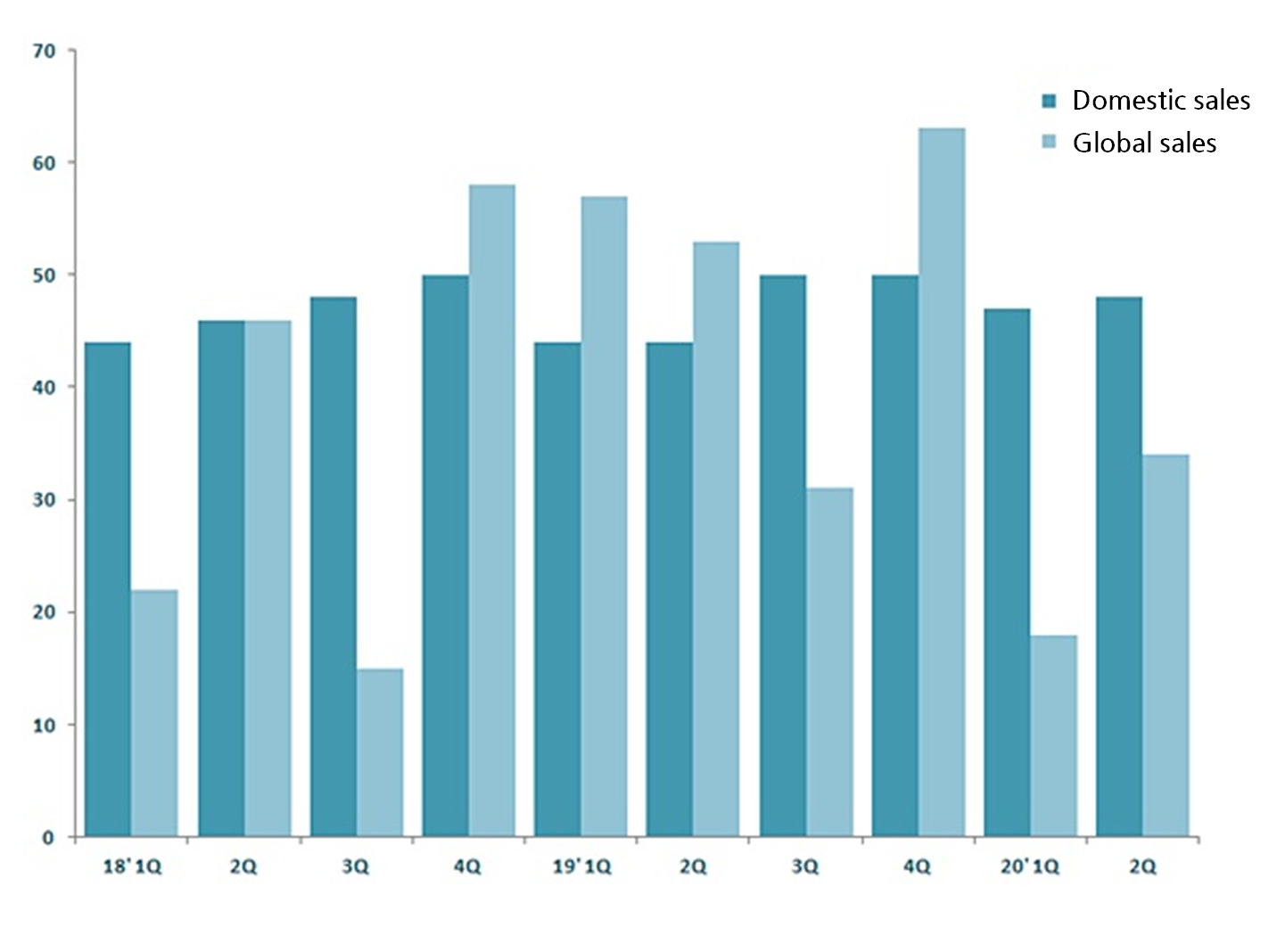
GC Pharma says Hunterase’s export in the first half of the year reached 5.2 billion won, which was 35.4 percent of the overall sales.
The drug’s export of 20.3 billion in last year surpassed the domestic sales of 18.7 billion won for the first time.
At the moment, GC Pharma supplies Hunterase to markets in Central America and North Africa, and it has been improving the drug’s commercial value by lately entering other global markets.
Early this month, GC Pharma won an approval by China’s National Medical Products Administration (NMPA) for Hunterase.
Care Pharma Hong Kong, a rare disease treatment specializing offshoot of Canbridge Pharmaceuticals, licensed out the development and commercialization exclusivity in China, Taiwan, Hong Kong and Macao regions and successfully passed the Chinese pharmaceutical authority after 20 months since the partnership deal in January last year.
The Korean company estimates there are 3,000 Hunter syndrome patients in China.
The drug is expected to significantly leverage the international sales as it was approved as the first Hunter syndrome treatment in China with a sizable market.
Soon, the drug would be knocking on the Japanese market as well.
GC Pharma’s partner, Clinigen K.K.
has submitted a New Drug Application in last March to Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) for their approval review on Hunterase Intracerebroventricular (ICV).
Hunterase ICV is administered to the brain directly to deliver the medicine to cerebral ventricles.
The specific administration route can overcome the limitation of other intravenous injection that cannot improve the cognitive improvement as the medicine fails to permeate through the blood-brain barrier (BBB).
The global Hunter syndrome treatment market only has a handful of competitors.
The limited size of the rare disease patients would ultimately hinder the domestic market expansion, but Hunterase is strategizing to rather consistently grow its global market presence in new overseas markets.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









