- LOGIN
- MemberShip
- 2025-12-27 00:23:57
- Different use different price for same substance drugs?
- by Eo, Yun-Ho | translator Byun Kyung A | 2020-10-15 06:06:20
One says “Why should drugs cost the same when their effects, target patient sizes and values are different?” Another argues, “Why should I pay more for a drug that shares a common substance with other drugs?” In some way, both of those arguments make sense.
And it is considered a conventional conflict of interest between a seller and a consumer.
An ‘indication-specific drug pricing’ means granting a pricing on a drug based on each indication with different value of innovation the drug owns to reflect the recent changes in drugs having various indications.
So far, an organization representing multinational pharmaceutical companies, the Korean Research-based Pharmaceutical Industry Association (KRPIA), has been focusing on expanding risk sharing agreement (RSA) and pharmacoeconomic evaluation (PE) exemption eligibility.
But now it is advocating differentiated pricing based on specific indication of the drug.
The industry trend of one drug having numerous indications has been apparent for at least five years, considering the South Korean market only.
Lately, various indications not only applicable for different lines of treatment, but also for different disease treatments have been added constantly.
But why now?
On Oct.
8, the industry was relieved to see a follow-on drug now eligible for RSA.
The revised regulation provides an option of RSA to cost-effective drug (latecomer) with treatment level equivalent to first-in-class drug.
However, the South Korean government also added another clause as well.
Although the regulation was alleviated on follow-on drugs, the government now demands for cost-effectiveness confirming evidence (administration cost comparison or PE) from a RSA drug when expanding its reimbursement regardless of having RSA-eligible indication or not.
After the said revision was disclosed, KRPIA officially addressed of ‘indication-specific drug pricing’ in an official statement submitted.
At the time, KRPIA claimed “The cost-effectiveness evaluation standard’s consistency and predictability of the final price could be improved, if the drug pricing was decided based on indication.
The labeled price could be kept the same, but the patients’ treatment access could improve if the contract is signed to apply differentiated indication-specific refund rate based on the actual cost-effective price for each indication.” At a glance, the indication-specific drug pricing and regulation to confirm cost-effectiveness in a drug seeking to expand RSA reimbursement, but it is far from it.
Previously, RSA-applied drug coverage expansion was processed by the Health Insurance Review and Assessment Service (HIRA) setting the reimbursement standard without reviewing cost-effectiveness evidence and the National Health Insurance Service (NHIS) negotiating based on increasing patient size and use volume and adjusting the refund rate.
Of course, nowadays the Cancer Deliberation Committee is the biggest hurdle for the pharmaceutical companies, but the said course of action was considered typical.
But basically, it means the latecomer drugs’ pricing are lowered with the same standard as the first-in-class drug, because the authority grants minimum pricing after comparing against alternative options’ administration cost and PE results.
And it creates a synergy effect when overlapped with latecomer’s drug approval, because the lowest pricing goes down as the number of follow-on drug listed with RSA goes up and listed indication expands.
In other words, the industry is complaining “Why should the drug pricing always fall and never get raised?
If the cost-effectiveness of an added indication is higher than the previously listed induction, then the pricing should be adjusted accordingly.”
The background of KRPIA and the industry’s claim is that the introduction of the indication-specific drug pricing in South Korea would highly likely to be limited to RSA-applied drugs (preferentially considered) that also adjust the refund rate.
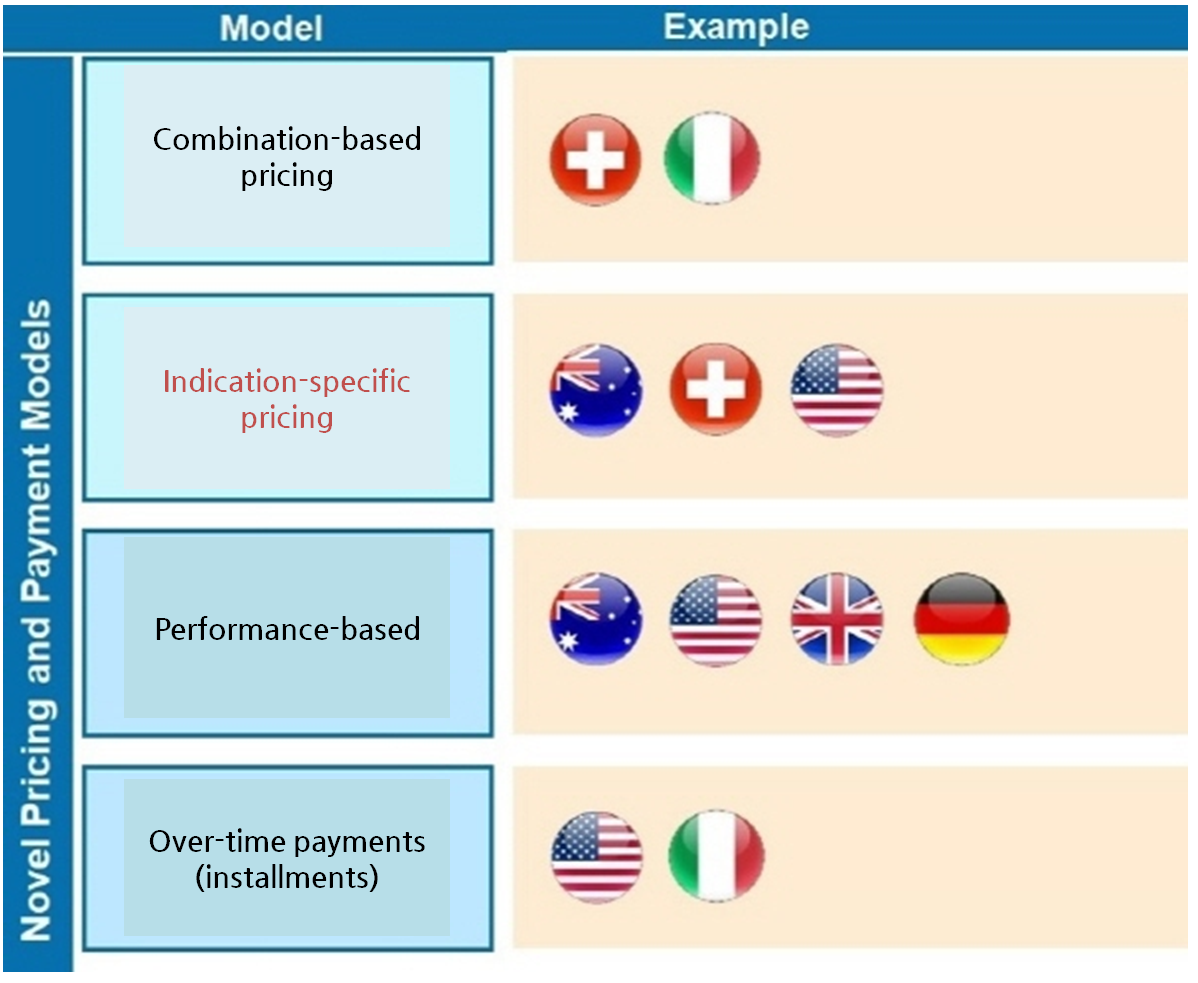
Currently, Australia, Switzerland and the U.S.
are using the indication-specific pricing that mostly maintains the initial labeled price but adjusts the refund rate.
And other types of novel payment models (NPM), such as combination-based pricing and over-time payments are used in Australia, Switzerland, the U.S.
and Italy to improve access to new drug.
The industry experts seem to agree the indication-specific pricing is the most suitable model in the South Korean market.
And from the government’s perspective, the notion is not to be dismissed blindly.
The self-explanatory term, ‘indication-specific pricing,’ literally means pricing a drug based on the value of each indication.
If the system, as described by KRPIA, adjusts the refund rate based on the value of the indication assessed through PE while maintaining the labeled price, the government could find more grounds to lower the drug pricing.
It is also important to note that most of new drug’s additional indications tend to be less innovative than their first indication.
The industry’s market access associate commented, “At this point, the reimbursement listing of a latecomer drug’s first indication may be easy, but growing number of companies has started to give up on listing additional indications with complications they face.
And now the patients are left behind only to watch their access to treatment get further out of reach.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









