- LOGIN
- MemberShip
- 2025-12-27 00:23:39
- Issues to look into when pricing drug by each indication
- by Eo, Yun-Ho | translator Byun Kyung A | 2020-10-17 06:37:47
Amending a regulatory system is not a simple work.
Moreover, introducing the indication-specific drug pricing would have to entirely change the premise of the National Health Insurance (NHI) system that grants ‘unified insured pricing on a single drug.’ Regardless of the final result, the government would need to have detailed discussions and survey the public opinion.
As for the industry, it is positive that the government is still open to the idea to discuss it further.
In fact, Vice Minister Kang Do-tae at Ministry of Health and Welfare (MOHW) spoke during an interview with the industry news media and noted, “The indication-specific pricing could help strengthening the treatment access in severe disease patients.
But the feasibility of realizing the novel payment system within the current billing structure and payment system the NHI system uses should be confirmed.
And also various views of stakeholders, such as related government bodies, pharmaceutical industry and civic group, should be sufficiently surveyed as well.” And there is already a number of issues raised regarding the subject.
Following are the concerned voices on adopting the indication-specific pricing;
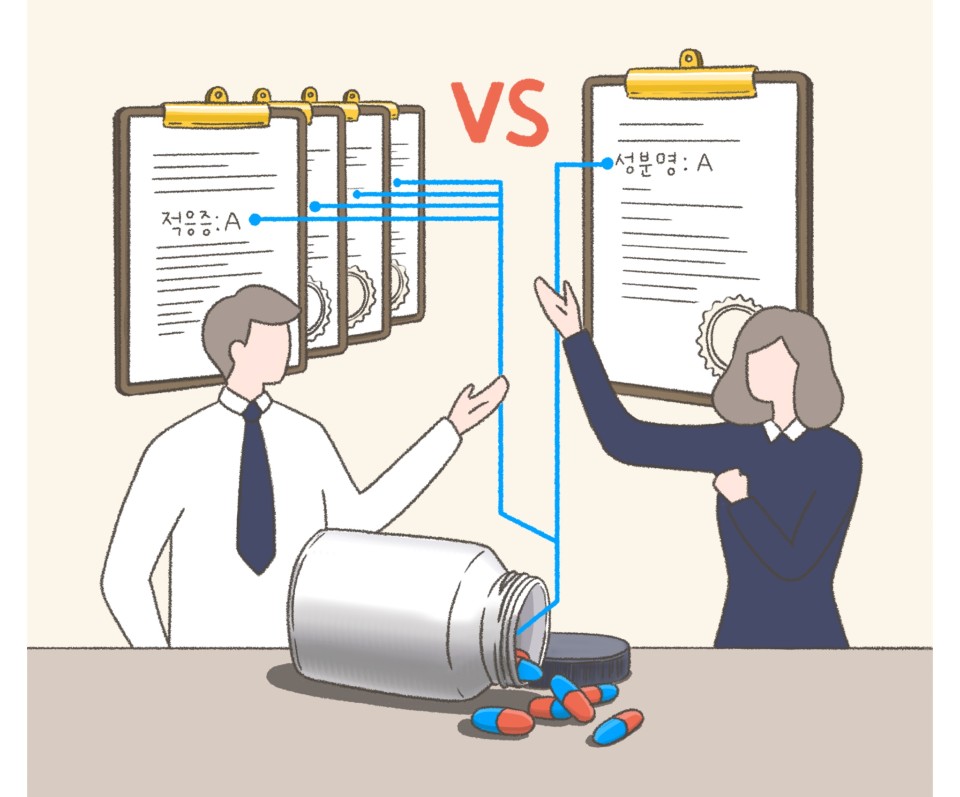
Using the indication-specific pricing would mean giving two to three unique codes on a single drug.
And the Health Insurance Review and Assessment Service (HIRA) and the National Health Insurance Service (NHIS) would undergo big changes in their billing system, which could cause confusion in healthcare institutes when inputting the main or sub disease code.
Nevertheless, these are literally some ‘inconvenience’ that can be handled.
When the government implemented the NHI coverage enhancement initiative and lowered the copayment rate of anticancer treatment expense to 5 percent and differentiated the billing, unique code for the variant copayment rate code was issued.
In other words, issuing unique code for each indication on a single drug could be bothersome, but not impossible.
However, the issue of the system abuse should be looked into.
For instance, a drug could be priced at 100,000 won or 150,000 won when prescribed as a stomach cancer or liver cancer treatment, respectively, according to the indication-specific pricing.
Then a healthcare institute may falsely report the drug as a prescription for treating liver cancer, when it was prescribed to a patient with stomach cancer to make profit from the difference.
Actually, there are drugs containing same substance but priced individually.
Regardless of the same substance, each item is approved under different name with a variety of doses and formulation.
An immunosuppressant Certican and anticancer treatment Afinitor both containing everolimus, and enlarged prostate treatment Proscar and hair loss treatment Propecia sharing finasteride are prescribed under different pricing.
Recently, Pfizer won separate approvals on Vyndaqel and Vyndamax sharing tafamidis and started the reimbursement listing procedure (Vyndamax).
But even with these drugs, there were reported cases of abusing the drugs.
People with hair loss receiving prescription of Proscar, instead of non-reimbursed Propecia, are apparently breaking the tablet into pieces to take them in smaller dose.
But considering the anticancer treatment prescription scene and the initial talks on indication-specific pricing limited to RSA drugs only, it would be unlikely for the system abuse to be apparent widely.
Nevertheless, the regulatory measures to manage such system abuse would be accompanied when implementing the indication-specific pricing.
An official from NHIS said, “Even if it is a same drug, a pricing reasonable in one country or payment model could be inappropriate in other countries.
Ultimately, it would be wise to select optimized payment model taking in account of unique situations in each countries.
We need to have talks on the possibility of realizing the novel payment system within the current billing data structure and payment system.”

Literally, the indication-specific pricing would differentiate the price of a drug a patient has to pay depending on the disease they have.
And for a cancer patient, it may be difficult to accept the fact that the specific cancer they are fighting against is costing them more.
In the end, the public should be aware and create a social consensus recognizing that the indication-specific pricing would ‘accelerate the reimbursement listing for anticancer treatment, and be a solution to drugs struggling to expand reimbursement on additional indication after its first.’ Patients could feel the burden of pricing, but ultimately receiving reimbursed treatment would be a better than no option at all.
Also the actual price is subject to change when adjusting refund rate, but the gap would be narrowed when the copayment rate is applied.
A market access personnel in a multinational pharmaceutical company urged, “Beyond the issue regarding the international reference pricing (IRP) system, the Korean offices are having more difficulties in getting the headquarters’ approval during the reimbursement expansion process.
A company has to give up on expanding the coverage when a drug’s actual price, despite the labeled price, is making a loss.
We want the public to understand that what the industry wants from the indication-specific pricing is not to burden the patients, but to improve treatment access for them.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









