- LOGIN
- MemberShip
- 2025-12-26 21:10:45
- How can the domestic drug price system be improved?
- by | translator Choi HeeYoung | 2020-11-17 06:28:32
As drugs that target specific genes or activate the immune system to treat diseases have been released, the use of one drug for various types of cancer has become more frequent.
As of 2018, 75% of target anticancer drugs are multiple indications, and in the case of immune anticancer drugs, thousands of single and combined clinical trials for various carcinomas are reached.
There are many opinions that the current drug price system needs to be changed.
This is because, as indications are added, only the factors that lower the drug price increase, reducing the patient's access to new drugs.
How can the domestic drug price system be improved?
To find the clues, Dailypharm held the 40th Future Forum on the 11th at the Moonjeong-dong office building under the theme of 'prerequisites for introducing drug prices by indications'.

Park Mi-hye, professor of the College of Pharmacy at Sungkyunkwan University, Choi Kyung-ho, secretary of the Department of Health and Welfare Insurance and Pharmaceutical Affairs Department, Lee Young-hee, head of the Drug Price System Improvement Department of the Health Insurance Corporation, and Ryu Chi-young, director of the Korea Global Pharmaceutical Industry Association (KRPIA), attended.
Prof.
Mi-Hye Park, who came out as a speaker on this day, analyzed the drug price system for each foreign indications under the theme of 'A measure of valuation when expanding indications of new drugs-Focusing on immune anti-cancer drugs and targeted anti-cancer drugs', and suggested the most suitable method for the domestic environment.
Currently, Korea is maintaining or lowering drug prices in consideration of the additional usage (additional financial requirements) of already approved drugs.
This is a method that recognizes only the first value assigned at the time of listing of the first salary and not the value of other indications.
For this reason, as the standard is expanded, the drug price gradually decreases.
Based on the standard anticancer drug, as the number of supplementary extensions increased, the tendency of drug price cuts was more pronounced, and as indications increased, the dropping rate was found to be greater.

Park pointed out that the current method, which does not recognize the value of additional indications, is also contrary to the 'value-based evaluation system', which determines whether or not to pay benefits based on relative clinical usefulness and cost-effectiveness when listing new drugs.
.This is because even if an unlicensed drug proves high value in a new indication, it is excluded
.There are also concerns about this
.Professor Park Mi-hye explained, "If the value of the additional indication is higher and it is not recognized as a drug price, there is a possibility that pharmaceutical companies will not promote the expansion of the standard." In the light of research by Cole et al., "If the value of each indication is recognized, high-value indications can receive high drug prices
.Therefore, pharmaceutical companies will be more active in research and development on new indications, so that patients' treatment options can be wider
.Therefore, social welfare can be further increased.” Differential structure of drug prices by actual indication, such as Australia and Italy How to determine reimbursement of multiple indication drugs abroad
?As a result of an analysis of the survey published this year by Professor Park by the OECD, it was found that there are no countries that explicitly operate the drug price system by indication, but some countries are implementing a structure in which the price of drugs by indications is substantially different for certain drugs
.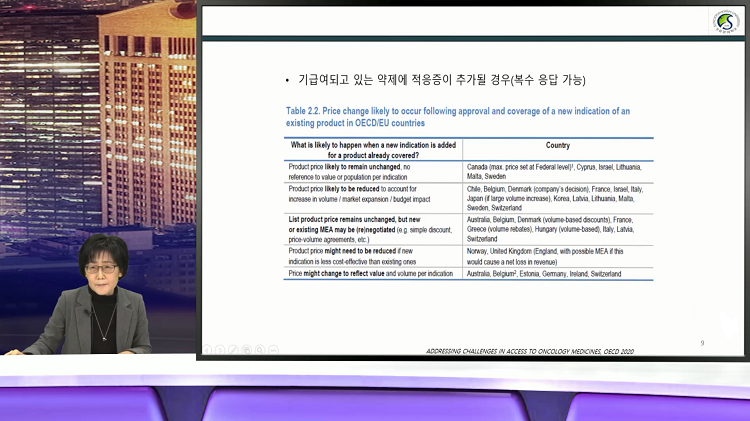
.Australia and Germany calculate single-weighted pricing for each indication, reflecting the cost-effectiveness of the patient group for each indication when determining drug prices for multiple indications
.In addition, Italy, Belgium, France, Switzerland, etc
.negotiate a risk-sharing system (RSA) for each indication, so that the drug price is substantially different
.Professor Park said, "Typically, Australia is making changes by calculating a single weighted average price by referring to the Medicare data collected during the prescription period." "Also, Italy has established a registry very well for nearly 20 years, so that which drug is suitable for any indication of a patient
.It is possible to track how much it has been used
.Based on this, different types of risk-sharing drugs are implemented for each indication even for the same drug." In addition, Italy, Belgium, France, Switzerland, etc
.negotiate a risk-sharing system (RSA) for each indication, so that the drug price is substantially different With the increasing number of anticancer drugs with multiple indications, what is the most appropriate drug price system considering the domestic environment
?Prof
.Park disclosed the results of the survey to practitioners related to drug prices of pharmaceutical companies registered with the Korea Global Pharmaceutical Industry Association (KRPIA), the Korea Biopharmaceutical Association (KoBIA), and the Korea Pharmaceutical Bio Association (KPBMA)
.System 3 is the most supported from▲ Calculation of a single weighted average price according to the value of each indication (system 1), ▲ application of a different risk-sharing system for each indication (system 2), and ▲ different drug prices for each indication (system 3)
.Professor Park said, "The majority of respondents evaluated the current system as negative in terms of improving access to new drugs and reflecting appropriate values, and more than 90% agreed with System 2, which received an overall positive evaluation in the direction of system improvement." He said that the way to give different drug prices is practically limited
.Based on this, Professor Park proposed to introduce a method that practically differentiates drug prices by applying different types of risk-sharing system for each indication, as in Italy
.Professor Park said, "In Korea, RSA is already being implemented, and since 2018, selective benefits have been introduced to medicines, and a system that differentiates the level of reimbursement for each patient has been established, so it seems to be a sufficiently accessible policy." Even when considering harmony, it is considered the most feasible solution." He added, "Currently, the RSA application is operated on a relatively strict basis, so it is difficult to apply it to all drugs for which indications are expanded
.This part requires coordination." Professor Park said, "In the future, the possibility of expansion of the indications for anticancer drugs is very high, but the potential risk is left alone
.If this situation is repeated, the damage will eventually be suffered by the patients
.It is time for a future-oriented policy alternative."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









