- LOGIN
- MemberShip
- 2025-12-26 21:10:22
- Prevenar and Sky Cellflu soar, Keytruda tops again
- by Chon, Seung-Hyun | translator Byun Kyung A | 2020-11-30 06:20:41
The novel coronavirus infection (COVID-19) has shuffled the top pharmaceutical market leader board.
While immunotherapy Keytruda topped the market again, the sales in a pneumococcal 13-valent conjugate vaccine Prevenar 13 and a flu vaccine Sky Cellflu 4 surged dramatically.
The sales in innovative new drugs like Tagrisso and Prolia also grew noticeably.
According to a pharmaceutical market research firm IQVIA on Nov.
25, MSD’s Keytruda generated 41.6 billion won in last third quarter.
Compared to the same quarter last year, the figure soared by 25.9 percent and topped the market for three consecutive quarters, pushing Lipitor down.

In the second and third quarters, Keytruda widened the gap with Lipitor.
At the third quarter point, Keytruda accumulated sales of 113.8 billion won, which was 8.6 billion won more than Lipitor with 105.2 billion won.
If the drug continues the trend, it is highly likely to take the top spot this year, when it marks the sixth year of launch.
Released to the South Korean market in 2015, Keytruda is an immune checkpoint inhibitor that blocks PD-L1 receptor from biding with PD-1 pathway on the surface of T-cell and activates immune cells to treat the tumor cells.
Immediately after the market release, Keytruda’s sales have remained around 3 billion won, but the figure grew rapidly since the second half of 2017.
The demand on the drug has surged as the healthcare insurance coverage was granted from August 2017 as a second-line therapy treating patients with non-small cell lung cancer (NSCLC).
Keytruda’s sales in the first quarter of 2018 surpassed 10 billion won, and climbed up to the 20 billion won-line in the following fourth quarter.
From the second quarter last year, it has reached the 30 billion won-line, and exceeded 40 billion won in this year’s third quarter.
The demand for Keytruda in the prescription market continued to grow amid COVID-19.
A pneumonia vaccine Prevenar 13 had an exponential growth.
In the third quarter, the vaccine made 24.2 billion won, doubling last year third quarter’s sales.
Prevnar 13 is a pneumococcal conjugate vaccine (PCV13) that prevents infection from 13 types of pneumococcal pneumonia (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F).
Provided to all age groups older than 6-week-old, the vaccines for adults are distributed by Chong Kun Dang, whereas Korea Vaccine is in charge of distributing vaccine for children and infants.
Prevenar 13’s sales usually peak in the fourth quarter, when typically the demand for vaccination surges, and drop in the first quarter.
This year’s first quarter sales grew more than the previous quarter, making 17.6 billion won, and its growth continued on for the second and third quarters, surpassing the quarterly sales 20 billion won-line for the first time.
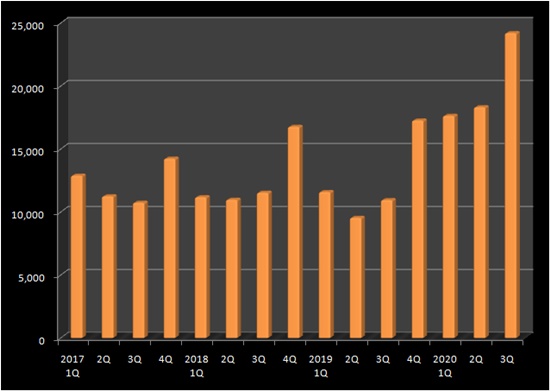
They evaluate the use of the vaccine in adults has leapt sharply as many were convinced by a number of experts claiming the vaccine can weakened the pneumonia symptoms induced from COVID-19, although it cannot prevent the pneumonia itself.
Besides Prevenar 13, Sky Cellflu 4 also entered the top ten as it generated 21.1 billion won amid COVID-19.
Unlike typical egg-based vaccine, the Korean-made quadrivalent vaccine is produced with top-of-the-line aseptic culture system, which does not require preservative.
As all flu vaccines for this year were quadrivalent and the supply for the vaccines increased with COVID-19, Sky Cellflu 4’s sales skyrocketed.
The amount of influenza vaccines distributed to the South Korean in this influenza season was about 29.5 million doses with an increase of 20 percent from last year.
The Korean company raised the Korean market distribution volume as it predicted the surge in flu vaccine demand due to COVID-19.
SK Bioscience shipped about 9 million doses with 70 percent jump compared to last year.
AstraZeneca’s anticancer treatment Tagrisso continued to generate significant sales.
The third quarter sales were 32.4 percent higher than last year, as it generated 27.9 billion won.
Tagrisso is a second-line treatment prescribed to NSCLC patients who developed tolerance in EGFR tyrosine kinase inhibitors (TKIs) like Iressa, Tarceva and Giotrif.
The medicine is considered a third-generation treatment that overcame EGFR-TKI tolerance.
After receiving the healthcare reimbursement from December 2017, Tagrisso has been growing consistently in South Korea.
The sales in the third quarter of 2017 marked 2.7 billion won, but it grew by tenfold in just two years time.
Amgen’s osteoporosis treatment joined the top ten by making 20 billion won in the third quarter.
Released to the South Korean market in November 2016, Prolia is a biologic osteoporosis treatment directed against receptor activator of nuclear factor-κB ligand (RANKL), which interferes with the formation, activation, and survival of osteoclasts.
The drug’s sales started taking off since it was listed for reimbursement in 2017 as a second-line treatment.
And Prolia’s sales exploded when it successfully expanded the reimbursement as a first-line treatment from April last year.
Also its sales partnership with Chong Kun Dang gave a boost as well.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









