- LOGIN
- MemberShip
- 2025-12-26 21:10:44
- Another effect of Botox, possible to treat hair loss
- by | translator Choi HeeYoung | 2020-12-21 06:19:23
Following cosmetic surgery, botulinum toxin formulations are expanding their scope to treat hair loss.
It is noteworthy as researches confirming the potential as a treatment for hair loss in Korea are ongoing.
In 22 subjects, Nabota was treated for 6 months and the reaction was confirmed.
As a result, the number of hairs increased statistically significantly at week 24.
The study results were published in the December issue of the SCI-level journal Journal of the American Academy of Dermatology (JAAD).
This study is a kind of exploratory study, and there is a limitation that there is no control group and the number of subjects is small.
However, Professor Park Byung-cheol said, "It is meaningful that we have confirmed the mechanism of hair loss treatment of botulinum toxin through a cell experiment, and that the researcher's clinical trial has confirmed its potential.
We can proceed to the later phase II clinical trial and the phase III study according to the results.
It was an opportunity to lay the groundwork for that,” he revealed the significance of the study.
Dailypharm conducted an interview with Professor Park to find out the potential of botulinum toxin preparations to treat hair loss.
▶What are the limits in the treatment of androgenetic hair loss? -The oral drugs Finasteride (Propecia), Dutasteride (Avodart) and minoxidil are the treatments for androgenic hair loss that have been approved by the US Food and Drug Administration (FDA) so far.
These are the most important treatments and the basis of hair loss treatment.
However, in some patients, the effectiveness of these treatments may be insufficient, and it is true that each drug has side effects.
In addition, Finasteride and Dutasteride have limitations that women of childbearing potential cannot take.
▶A study on hair loss treatment was conducted with botulinum toxin.
Please explain the research background, design, and results. -Botulinum toxin is used not only to improve wrinkles but also to prevent and treat scars, and one of the main mechanisms at this time is to inhibit TGF-β.
TGF-β is also known to inhibit hair growth and progression from hair to telogen hair.
I thought there would be something in common, so I started my research.
This study was a pilot study, comparing before and after treatment in 22 subjects without a control group.
Except that there was no control group, the reaction was confirmed after treatment for at least 6 months following the general guidelines for confirming the improvement in the efficacy of hair loss suggested by the Ministry of Food and Drug Safety.
Effectiveness evaluation statistically verified the change in the number of hairs per unit area at 12 and 24 weeks compared to the baseline at week 0 and the degree of improvement by clinical pictures.
Side effects were confirmed through vital signs at each visit, medical examination, and blood tests at weeks 0 and 24.
The treatment method was to inject botulinum toxin (Nabota) into the hair follicle of the subject every 4 weeks with 30 units of botulinum toxin (Nabota) for a total of 24 weeks for a total of 24 weeks.
At week 24 of treatment, the number of hairs increased significantly (p=0.012).
No serious drug-related adverse events were reported.
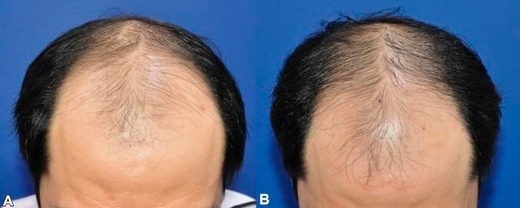
△ Overall scalp shape improvement, △ improvement of hair density in the area of hair loss, △ improvement of the degree of hair loss, △ improvement of hair growth rate, △ improvement of hair thickness.
▶What mechanism of botulinum toxin preparations has the effect of treating hair loss? -It is assumed that botulinum toxin inhibits the secretion of TGF-β1 from hair follicle cells, which inhibits the progression and growth of hair at rest.
The increase in TGF-β1 secretion by'dihydrotestosterone (DHT)' is known to be one of the major causes of androgenic hair loss.
In this study, an increase in TGF-β1 was also observed by DHT, and it was confirmed by cell experiments that the expression was decreased when treated with botulinum toxin.
▶Looking at the results of this study, there was a significant improvement at week 24, but not at week 12 (p=0.803).
In other words, it means that the change was insignificant until 3 months of the procedure.
What do you think is the reason? -I think the cycle of hair or the cumulative concentration of the drug may have had an effect.
Normally, the hair growth period is 4-6 years, and the period of regression is 3 weeks, and the period of rest is about 3 months.
Considering this, it can be interpreted that it may not be effective at the third month.
Or it is thought that a period of cumulative concentration may have been necessary for the drug to exert its effect.
Accordingly, a follow-up study currently underway is confirming the treatment response by increasing the concentration slightly.
▶This study was only for men.
Could female hair loss patients benefit from hair loss treatment with botulinum toxin? -In fact, there are few treatments for female type hair loss, so many people are also interested in this.
Although clinical studies have not been conducted yet, the results cannot be predicted, but since TGF-β1 inhibits general hair growth, it is expected that botulinum toxin that blocks this will be effective in female-type hair loss.
Currently, botulinum toxin is also injected into female hair loss patients with consent at the clinic site for therapeutic purposes.
Since hair loss treatment is also a local treatment, it has the advantage that it can be treated while minimizing systemic side effects.
In particular, in existing wrinkles, injections are made into muscles with abundant blood supply, but in hair loss, because dermal hair follicle injections are performed, systemic absorption through blood vessels is expected to be less, so there is a high possibility that no noticeable systemic side effects were observed.
Since this study was only conducted for 6 months, long-term observation is necessary.
Problems have also been raised about the formation of neutralizing antibodies and resistance to botulinum toxin injections, but this is known to occur mainly in patients who frequently receive high-dose intramuscular injections.
Hair loss treatment is expected to have less resistance, but this part will be confirmed through follow-up studies.
▶Aren't patients reluctant to get an injection into the scalp? -Because getting an injection into the scalp itself causes pain, there is a factor to be reluctant.
However, since there is a motive for the treatment of hair loss, they are injected once a month, and various tips are used to minimize pain during injection, so most patients at the actual clinic say that they have pain, but it is acceptable.
▶Wouldn't the temporary effect of botulinum toxin formulations be a disadvantage?
What new treatment benefits do you think can offer more than existing hair loss treatments?
-Androgenic hair loss is basically a progressive disease caused by genetic factors, so if you stop taking any medication, hair loss may progress again, and this is the same with botulinum toxin treatment.
However, in this study, there are cases in which it was unofficially confirmed that hair loss does not progress for about 3 months after the last treatment.
Factors that cause hair loss can vary, and the effects on hair loss treatment are also diverse.
Accordingly, botulinum toxin can be used in a variety of ways, including the purpose of increasing the effect in patients with insignificant effect on the existing treatment, the purpose of maximizing the effect in patients with good effect in the past, and alternative purposes in patients with no effect on treatment.
.In particular, it is expected that it can be used in patients who are unable to take existing drugs
.▶Please tell us about the significance and limitations of this study and the follow-up studies This study is meaningful in that it has confirmed its possibility as an exploratory study as the first step to find out whether botulinum toxin can be used as a hair loss treatment
.In particular, the mechanism was also confirmed through cell experiments
.Since it is a small-scale study without a control group, it can be recognized as a universal treatment only when the later phase 2 and final phase 3 clinical trials are completed, but it is meaningful in that the basis for these studies has been prepared
.As a follow-up study, a study is underway to find the optimal concentration and usage to maximize the therapeutic effect
.In the future, we plan to study the therapeutic effect in gynecomastia, the presence or absence of neutralizing antibodies that may occur during treatment, and further analysis of the therapeutic mechanism at the cellular and hair follicle organ levels.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









