- LOGIN
- MemberShip
- 2025-12-26 21:10:48
- Technology exports are active despite COVID-19 outbreak
- by An, Kyung-Jin | translator Choi HeeYoung | 2020-12-28 06:14:00
The world is struggling with the surprise attack of COVID-19, but domestic pharmaceutical and bio companies' new drug technology export performance is very active.
SK Biopharm received the most down payment for the second consecutive year as it transferred the copyright to the Japanese region for a new epilepsy drug developed with its own technology.
Yuhan and JW Pharma have made the achievement of transferring new drug technology to overseas pharmaceutical companies for the third consecutive year since 2018.
The share of technology exports from bio-ventures with platform technologies such as Alteogen and LegoChem Biosciences has increased significantly.
◆ Bio-venture activities and new drug technology exports are '4 out of 5'
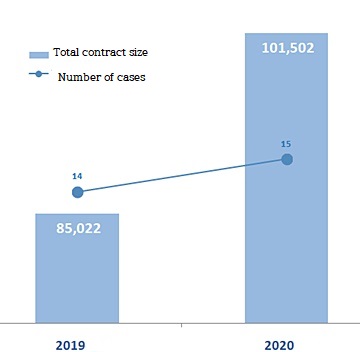
Compared to last year, when 12 companies won 14 contracts, the number of contracting companies decreased by two, but the number of contracts increased by one.
Technology exports are estimated to be ₩10 trillion, up 19.4% from last year.
The driving force behind the breakthrough of ₩10.15 trillion in technology exports is bio-ventures, while the world is in a chaotic state due to COVID-19 outbreak.
In April, LegoChem Biosciences signed a contract with Iksuda Therapeutics in the UK to transfer the original technology of antibody-drug complex (ADC) worth About ₩496.3 billion.
ADC (Antibody-Drug Conjugates) is a technology that enhances therapeutic effect by accurately delivering drugs bound to antibodies to antigens.
A total of five contracts signed by LegoChem Biosciences for a year using original technology.
In May, the company passed an ADC-based anticancer drug to Iksuda and won an additional contract worth $227 million, and in October, it signed a technology export contract with CStone Pharmaceuticals in China for up to $363.5 million for ADC anticancer drugs.
In December, it signed a technology export contract with Pyxis Oncology in the US and two pharmaceutical companies in Japan, respectively, related to ADC anticancer drugs.
In the case of transferring the copyright of three ADC-based anticancer drugs to Takeda subsidiary Millennium Pharmaceuticals in March last year, it means that six contracts were signed with the same technology.
In addition to LegoChem Biosciences, relatively small-scale bio-ventures showed outstanding technology export performance.
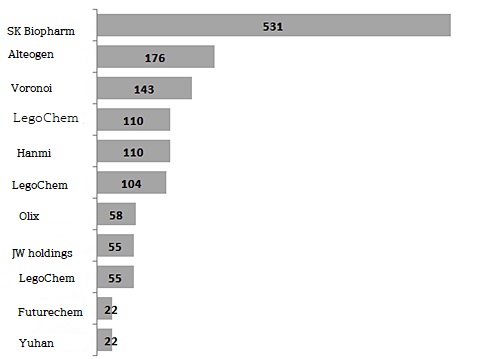
12 out of 15 new drug technology transfer contracts signed by domestic companies this year are achievements made by 7 bio companies including LegoChem, Futurechem, Alteogen, OliX, Voronoi, Genexin, and SK Biopharm.
Alteogen received $16 million as a down payment when it passed the right to use the source technology for Hybrozyme to 10 global pharmaceutical companies in June.
The technical fee for each stage according to clinical development, marketing authorization, and sales performance amounts to a total of $3.8 billion.
Alteogen's original technology for Hyaluronidase is a technology that enables large amounts of subcutaneous administration of all biologics administered intravenously.
By taking advantage of the scalability of the original technology that can be applied to various new drug development processes, it achieved an additional contract conclusion in 7 months following last November.
Futurechem signed a contract for a total of €1.22 million with IASON GmbH in Austria in May for the diagnosis of prostate cancer, followed by a technology transfer contract worth about ₩650 billion with HTA in China in September.
In addition, it is counted that OliX, Voronoi, and Genexine have been handed over.
◆SK Biopharmaceuticals No.
1 in contract payments, JW's outstanding global technology export for 3 consecutive years Among the technology transfer contracts signed this year, SK Biopharm's epilepsy treatment Xcopri (Cenobamate) received the largest down payment.
SK Biopharm secured approximately ₩54.5 billion in deposit without obligation to return when it signed a technology transfer contract with Ono Pharm.
The milestone according to the achievement of permission and commercialization is up to about ₩524.3 billion.
After commercialization, sales royalties of 10% or more were guaranteed.
SK Biopharm secured a down payment of ₩54.5 billion this year, the eighth largest of the domestic pharmaceutical industry.
SK Biopharm also signed a total of $530 million worth of Xcopri contracts with Swiss Arvelle Therapeutics last year.
The down payment secured at the time was $100 million, which is the third largest in history.
It has maintained the No.
1 position in technology export contract payments for two years in a row by being recognized for the value of being approved by the US Food and Drug Administration (FDA) as first-in-class with a new mechanism.
It has signed a technology export and supply contract for Winuf, a comprehensive nutrient solution, with Shandong Luoxin Pharmaceutical, a subsidiary of Luoxin Pharmaceutical in China.
The total down payment is $39 million.
The down payment without obligation to return is $5 million, which accounts for 12.8% of the total down payment.
Winuf is a three-chamber comprehensive nutrient solution that can be easily mixed and used with three ingredients such as lipid, glucose and amino acids by dividing one container into three.
It is composed of four lipid components, such as refined fish oil (20%), refined soybean oil (30%), olive oil (25%), and MCT (25%), as well as glucose and amino acids.
It is known that it contains a higher content of refined fish oil than existing products, so it contains more omega 3 and omega 6 fatty acids that promote the improvement and recovery of patients' immunity.
Previously, there was no case of commercialization of a third-generation comprehensive nutrient solution containing refined fish oil in China, and JW Life Science signed a high-purity contract with a high proportion of down payments as it was recognized for the distinction that JW Life Sciences is in charge of producing finished products after Winuf in China.
It is an evaluation that it was possible.
It has signed a global technology export contract for 3 consecutive years, starting with the technology transfer contract for atopic dermatitis new drug with Leo Pharma in 2018.
◆SK Biopharmaceuticals No.
1 in contract payments, JW Group's outstanding global technology export for 3 consecutive years The technology transfer contract between Hanmi and Yuhan is also a meaningful R&D achievement this year.
Hanmi transferred the GLP-1-based dual agonist returned from Janssen back to MSD within one year.
It is a dual-acting treatment that simultaneously activates GLP-1, which helps secretion of insulin and suppresses appetite, and glucagon, which increases energy metabolism, and the original technology of Hanmi Pharmaceutical's LAPSCOVERY, which increases the duration of the drug, was applied.
The contract fee, which is not obligated to return, is $10 million, and the maximum contract size, including staged technical fees (milestone), is $870 million.
It was positively evaluated for discovering new potential in the field of non-alcoholic steatohepatitis (NASH) instead of diabetes and obesity, which were the existing indications, leading to a new contract.
Yuhan made a turning point by handing over gastrointestinal disease treatment candidates, which had been pending for nearly two years, to a small overseas bio-venture without obtaining satisfactory results in the domestic II clinical trial.
Yuhan received $2 million of Procesa shares as a down payment by handing over the global copyright of the new functional gastrointestinal disease drug YH12852 to Processa Pharmaceuticals.
The maximum contract size, including staged technology fee (milestone), is up to $410.5 million.
After commercialization, it was agreed to receive a certain percentage of net sales as royalties.
Processa Pharmaceuticals, a partner, will be listed on Nasdaq after the signing of the contract, and is looking forward to further development, saying that it will pursue a phase II clinical trial in the US early next year.
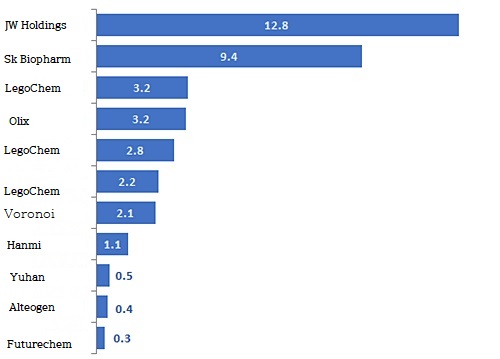
While the overall contract size increased, the proportion of down payments decreased relatively as option contracts and comprehensive platform technology contracts for which the inflow of subsequent milestones was uncertain.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









