- LOGIN
- MemberShip
- 2025-12-26 17:26:03
- Stepped pricing to put pressure for patent challenge
- by Kim, Jin-Gu | translator Byun Kyung A | 2021-01-14 06:16:28
The stepped drug pricing system, revived since last July, seems to have affected the pharmaceutical industry’s patent strategy.
The pricing system would create a structure to naturally diminish the drug pricing competitiveness in other companies, when 20 or more companies simultaneously and successfully challenge the patent.
Pharmaceutical companies’ patent strategy originally focused on winning the preferential sales right, but the revised pricing system would unfold even more competitive patent challenge scene as the success would not only bring the preferential sales right but also more favorable generic pricing.
Some complain now the companies are inevitably pressured to challenge the patent to defend the generic competitiveness.
◆Patent challenge inevitable to claim better generic pricing According to the pharmaceutical industry source on Jan.
14, the pharmaceutical companies are to face heated competition to nab the generic approval first as the stepped pricing system has been enforced from last July.
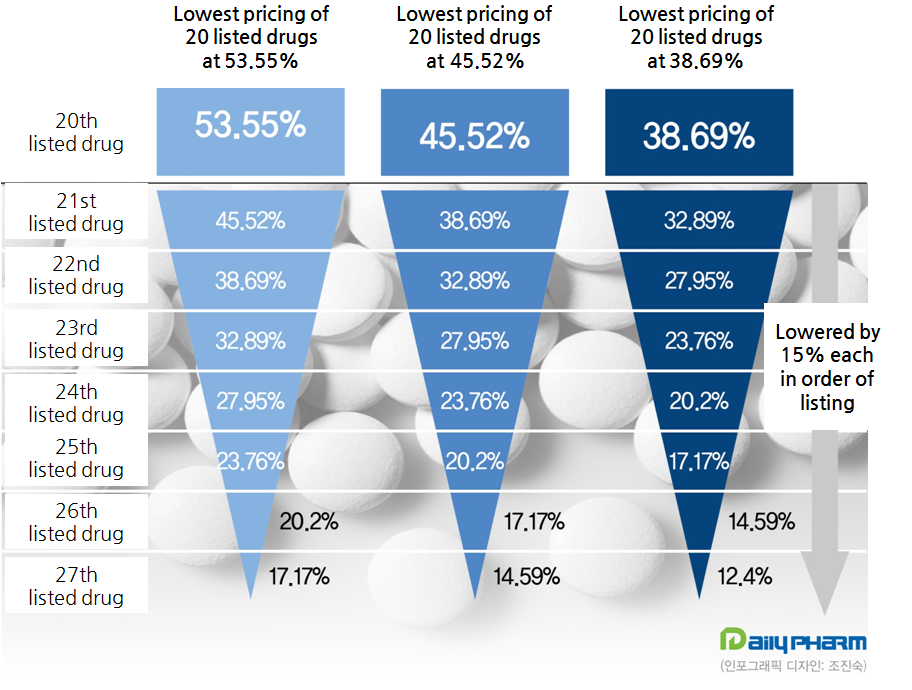
The core objective of the system is to differentiate the pricing after listing a 20th product with a same substance.
53.55 percent of the original’s pricing would be applied on up to first 20 drugs to be listed.
And other drugs listed afterwards would be applied with pricing lower by 15 percent each.
In other words, up to 19th generics, except for the one original drug, would be able to receive the pricing level of the upper limit.
Considering the reimbursement listing is decided once every month, the stepped pricing system has been technically extended to a first-come-first-served approval competition.
The approval competition would be passed straight on to patent challenge competition.
As for pharmaceutical companies, the regulatory change has increased the benefit in patent challenge.
Previously, the benefit was limited to earning a preferential sales right, but now it can also grant the top pricing.
But in the same sense, a company that did not challenge the patent would have to market their generic later and cheaper than others.
◆49 generic makers challenged Jardiance patent when new pricing system emerged Some say the patent challenge scene has gotten saturated already due to the revised pricing system.
Eventually, the ruling was in favor of 50 generic companies.
The interesting part of the proceeding was the timing of these patent challenge cases.
Most of the companies filed the patent litigations after April 2019, when the framework of the stepped pricing system surfaced.
Starting from Chong Kun Dang in March 2015, 12 companies requested for the patent nullification, in which all of them lost.
Three years later, Chong Kun Dang retried and requested for the negative confirmation of the scope in January 2018.
The Korean company was alone during the legal case, but it won in May 2019.
From June 2019, other generic makers tried their luck.
49 companies challenged the exact same patent as of August last year.
The industry notes the swarm of patent challenge rushed in after the stepped pricing regulation came in sight.
The majority of them were shooting for the ‘good pricing.’ A pharmaceutical industry insider explained, “Usually the patent challenge is filed within 14 days from the day of first case registration to win the preferential sales rights.
But for Jardiance patent, the tens of companies jumped into the patent challenge a year and a half after Chong Kun Dang began the patent challenge.” He added, “It could be that some tried after Chong Kun Dang’s success, and some joined the legal suit to not fall behind in the pricing competition.” ◆Patent challenge could mean nothing if generic not developed before patent expiration Regardless of increased benefit in challenging a patent, some raise the voice of concern.
Small and medium enterprises without the capacity to challenge the original patent argue the regulation is cutting them out of pricing competition.
25 companies have challenged AstraZeneca’s antiplatelets Brilinta (ticagrelor) and received the preferential sales rights before the patent expires late this year.
The preferential sales would be protected from November this year to August next year.
Assuming the 25 companies would all launch their products, other generics to be released to the market after August next year would receive already reduced pricing.
And there is no guarantee of the maximum pricing to all who successfully challenged the original patent overcoming all difficulties.
These companies can launch their generics after April 2023.
But if one of them fails to finish developing their generic by April 2023, any of other 25 companies that evaded the product patent can launch their generic from January 2024.
Regardless of all resources put into the legal case, the company would lose the upper limit pricing when their generic is not ready to be launched when the patent is expires.
A pharmaceutical industry insider said, “The patent challenging competition was initially intense to begin with for the sake of preferential sales right.
But exclusivity in the market for nine months and receiving lower pricing are quite different story.
The generic makers would now have to engage in even more fierce competition centering patent challenge.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









