- LOGIN
- MemberShip
- 2025-12-26 17:26:03
- NOAC prescription market growth stagnates
- by Kim, Jin-Gu | translator Byun Kyung A | 2021-01-27 06:15:26
The novel oral anticoagulant (NOAC) market seems to be plateauing after enjoying consistent two-digit growth for years.
The outpatient prescription market marked a growth of almost 20 percent until 2019, but the growth shrunk down to 3 percent last year.
Regardless of the decreased growth, Lixiana is still topping the market.
Except for the market leader, the prescription performance by Xarelto, Pradaxa and Eliquis have dropped.
The generics entering market seems to have critically affected Eliquis making a negative growth for the first time last year.
Its generics are expanding their market presence from the second quarter in 2019.
◆NOAC market grows 3 percent making 184.3 billion won According to pharmaceutical market research firm UBIST on Jan 25, overall the last year’s NOAC market generated 184.3 billion won with a growth of 3 percent from the year before.
The overall market volume expanded, but the growth was sluggish compared to previous years.
Since the launch of Xarelto in 2009, the NOAC market welcomed Pradaxa and Eliquis in 2013, as well as Lixiana in 2016.
Replacing the older generation anticoagulant warfarin, the NOAC rapidly got bigger.
In last six years, the NOAC market generated 34.5 billion won, 80 billion won, 114.9 billion won, 151.5 billion won, 179 billion won and 184.3 billion won in year 2015 through 2020, respectively.
Its streak of two-digit growth was crippled last year with 3 percent, after seeing 132 percent, 44 percent, 32 percent and 18 percent growth in 2016 through 2019, respectively.
◆Lixiana growth by 7 percent, when Xarelto, Eliquis and Pradaxa drop The market’s star items showed contrasting performance.
Only the prescription volume of Lixiana by Daiichi Sankyo expanded, when all three others underperformed.
Generating 59.9 billion won and 64 billion won in 2019 and 2020, respectively, the Lixiana prescription volume had a 7-percent growth.
The drug may have been the last to join the market, but it topped the market in 2019 and is still widening the gap with the runner-up.
The pharmaceutical industry analyzes the co-promotion deal signed with Daewoong Pharmaceutical is the key to the growth surge.
In December of 2015, right before the product launch, Daiichi Sankyo inked the Lixiana co-promotion contract with Daewoong Pharmaceutical.
The contract is still effective today.
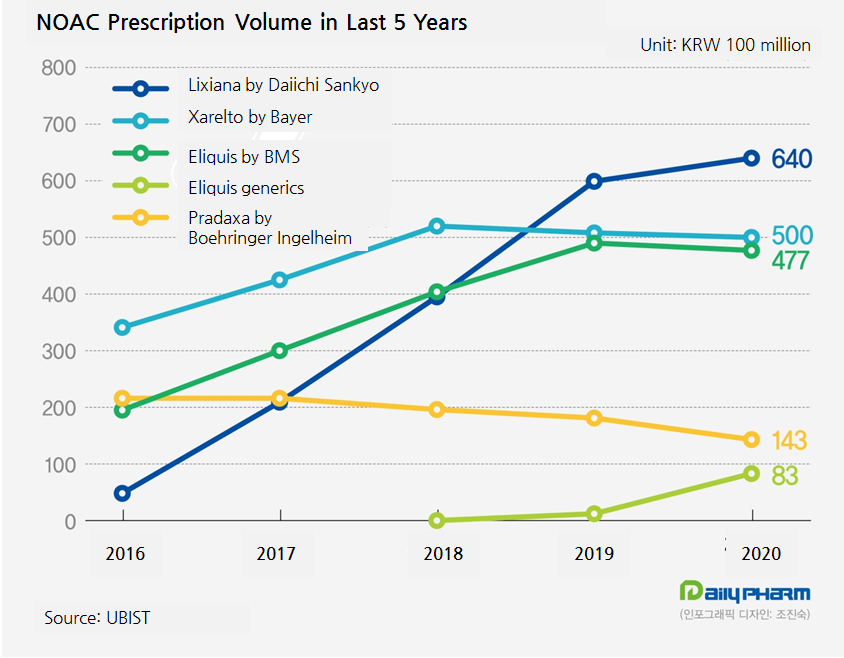
Its prescription volume peaked in 2018 with 52 billion won, the volume has been on a downhill since 2019 making 50.8 billion won.
Last year the drug’s volume dropped again to 50 billion won.
The industry experts project it would go under 50 billion won this year.
Eliquis also continued to make growth until 2019, but it turned downward last year.
In year 2016 through 2020, the drug’s prescription volume hit 19.5 billion won, 30 billion won, 40.4 billion won, 49 billion won and 47.7 billion won, respectively.
The industry experts point out the generics is the culprit of the plateauing growth.
Since June last year, generic versions of Eliquis like Chong Kun Dang’s Liquisia, Yuhan’s Yuhan Apixaban, Samjin Pharm’s Elxaban, Hanmi Pharamceutical’s Apixban, Aju Pharm’s Eliban and YooYoung Pharmaceutical’s Yupix have been released to the NOAC market.
The total prescription volume of Eliquis generics surged from 1.2 billion won in 2019 to 8.3 billion won in 2020.
In last year, Liquisia, Elxaban and Yuhan Apixaban respectively generated 2.6 billion won, 1.7 billion won and 1.1 billion won.
Taking account of the inclining quarterly prescription volume, the experts project they would break through the 10 billion won mark this year.
Since 2016, Pradaxa’s prescription volume has been dropping.
The volume plummeted to 14.3 billion won last year.
Compared to 18.1 billion won in 2019, it took a 21-percent drop.
Boehringer Ingelheim signed a co-promotion deal with Boryung Pharmaceutical from 2018, but it has not been so effective.
◆Generic versions of Xarelto and Pradaxa ready for launch And in this year, Xarelto and Pradaxa’s generics are to be released to the market and accelerate the shift in the NOAC market.
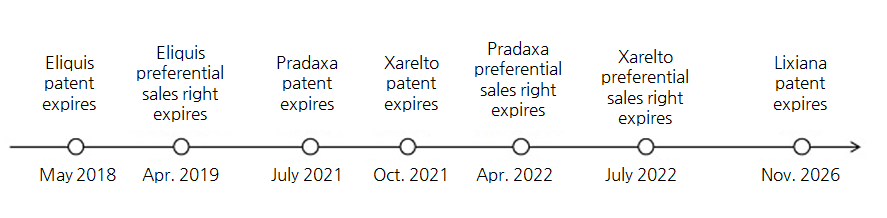
Intro Bio Pharma’s Dabican, Aju Pharm’s Dabitran, Jinyang Pharm’s Pradabi and Huon’s Hubitran are preparing for the launch.
The four companies won the patent dispute against the original drug company Boehringer Ingelheim in January 2019.
The obtained preferential sales rights are to last until April 2022.
Another NOAC, Xarelto is also expecting its generic versions—Hanmi Pharmaceutical’s Riroxban and SK Chemical’s SK Rivaroxaban—to emerge in October.
If Chong Kun Dang, successfully evades the product patent before Oct.
4, it would be able to market the product even before Hanmi Pharmaceutical and SK Chemical regardless of the preferential sales rights.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









