- LOGIN
- MemberShip
- 2025-12-26 17:26:02
- More options for ankylosing spondylitis including Taltz
- by | translator Byun Kyung A | 2021-02-09 06:04:49
Besides the first-in-class Cosentyx (secukinumab), Taltz (ixekizumab) was released to the South Korean market with its strong evidences.
Some specialists predict the guideline, initially recommending the drug to be used after using tumor necrosis factor-alfa (TNF-α) inhibitor, could be changed.
One of the most frequently diagnosed rheumatic diseases, ankylosing spondylitis mostly uses a nonsteroidal anti-inflammatory drug (NSAID).
But a biologic drug is recommended for patients with high disease activity.
Humira (adalimumab) and Remicade (infliximab) are TNF-α inhibitors, a most commonly used biologic treatment type.
Although the drugs are recommended first with their credible amount of clinical data, 30 percent of the patients apparently do not respond to TNF-α inhibitor.
Professor Hong Seung-jae at Kyung Hee University Medical Center (Department of Rheumatology) says only one out of three patients maintain the treatment, two years after using biologic treatment.
40 percent of the patients suspend the treatment before starting the second cycle, because of insufficient efficacy.
And now IL-17A inhibitors are emerging as a new option.
16 years after a first TNF-α inhibitor was approved, Novartis’ Cosentyx was released as a new drug to treat patients with ankylosing spondylitis.
And Eli Lilly’s Taltz followed with a same indication, and joined the competition in the South Korean market as received the National Health Insurance (NHI) reimbursement in last October.
Taltz’s strengths in treating ankylosing spondylitis are exceptional level of efficacy and safety.
To confirm the efficacy and safety in treating the disease, the COAST-V study had patients who are biologic disease-modifying antirheumatic drug (bDMARD)-naïve, and the COAST-W study had patients who previously had an inadequate response or were intolerant to TNF inhibitors.
Both studies chose more stringent primary endpoint, unlike other preceding clinical trials on ankylosing spondylitis, which was the Assessment of Spondyloarthritis International Society 40 (ASAS40) response showing over 40 percent improvement in the patients’ symptoms.
The trials were the only and first studies to have set ASAS40 as a primary endpoint.
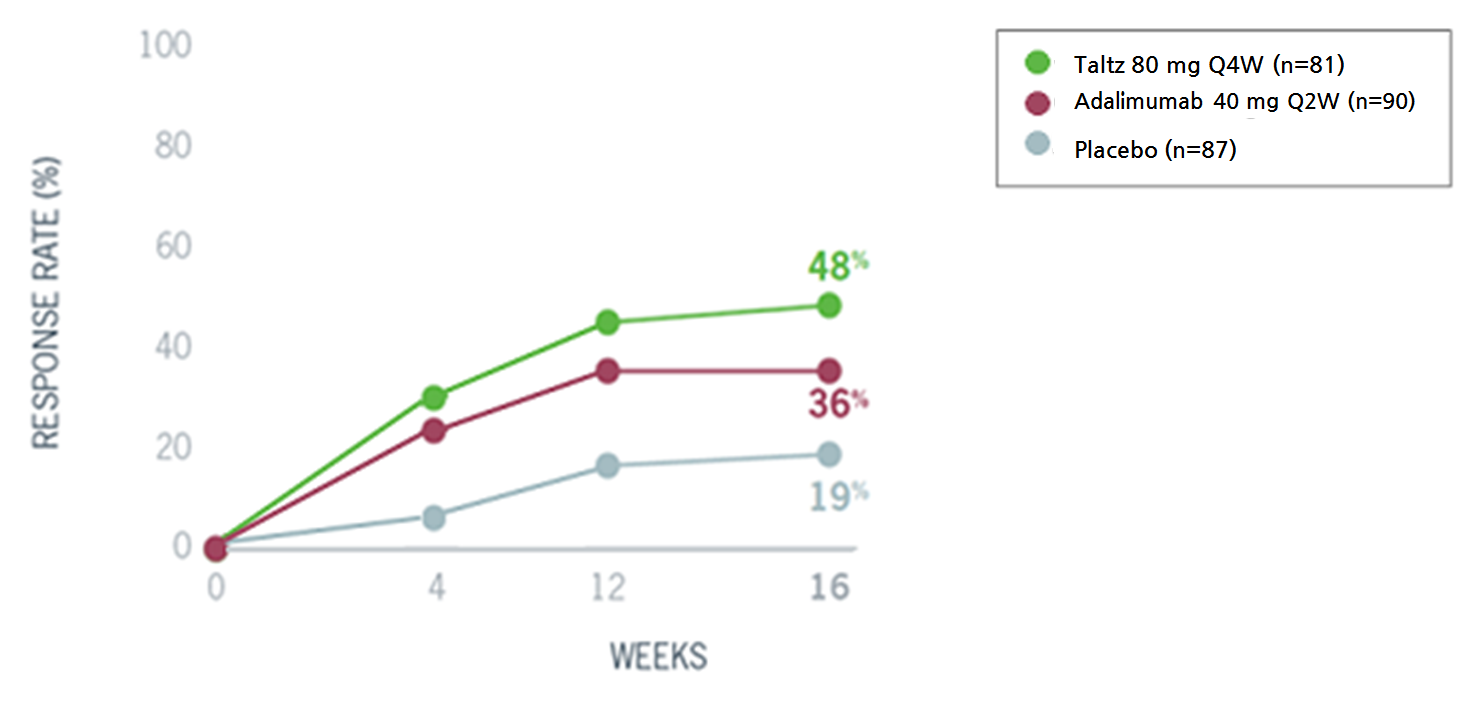
The results indicated statistically meaningful improvement compared to the placebo group.
Taltz also resulted in statistically significant outcome in secondary endpoints—objective signs of inflammation as indicated by elevated C-reactive protein (CRP) and/or magnetic resonance imaging (MRI), as well as patient-reported outcome (PRO).
And a head-to-head COAST-V study compared the drug against a TNF-α inhibitor Humira, frequently used to treat patients with ankylosing spondylitis.
Ultimately, the Taltz patient group showed higher response rate compared to the Humira patient group.
Moreover, Taltz has notable safety profile.
A TNF-α inhibitor is banned on patients with severe heart failure, whereas Taltz can be used regardless of the underlying condition.
Professor Hong Seung-jae elaborated, “Once-biweekly administered Taltz is more convenient than other once-weekly drugs, and it is known for its high ASAS40 response proving the efficacy.
And because it does not have the safety issue the TNF-α inhibitors, the drug can be administered to patients with heart failure.” When the clinical records of Taltz accumulate in the future, the professor thinks the reimbursement and treatment guideline can be easily changed.
Currently, Taltz is indicated for use in both first and second-line treatment, but the NHI reimbursement is granted only for using when a patent shows no response to a TNF-α inhibitor.
Also, the guideline by the Assessment of SpondyloArthritis International Society/ European League against Rheumatism (ASAS/EULAR) recommends using TNF-α inhibitor with more extensive clinical data, and then recommends an IL-17A inhibitor if the patient does not react to the TNF-α inhibitor.
Professor Hong expressed his anticipation in the prospective changes in the reimbursement standards, and said “The healthcare reimbursement standard and overseas guideline categorize the drug as a second-line treatment, as it lacks sufficient amount of clinical data in South Korea.
But the data is accumulating globally, and some experts claim the drug should be used at a same level with TNF-α inhibitor.” Meanwhile, relevant experts project other biologic treatment could become a variable in the expansion of an IL-17A inhibitors in the ankylosing spondylitis treatment market.
Currently, various Janus kinase inhibitors, such as tofacitinib and upadacitinib, are in process of conducting clinical trials targeting ankylosing spondylitis.
Specifically, upadacitinib (brand name Rinvoq) was indicated in EU to treat patients with ankylosing spondylitis, showing off its intention to join the scene.
Unlike other biologic treatments, orally taken JAK inhibitor could be preferred more.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









