- LOGIN
- MemberShip
- 2025-12-26 17:25:58
- Despite COVID-19 and NDMA metformin prescription surges
- by Chon, Seung-Hyun | translator Byun Kyung A | 2021-02-10 06:04:14
Metformin-based antidiabetic drugs showed exceptional growth in the prescription drug market.
Both metformin combination and single agent drugs expanded their prescription volume and maintained the credibility in the clinical scene, regardless of the novel coronavirus disease (COVID-19) outbreak and impurity contamination incident.
On Feb.
9, a pharmaceutical market research firm UBIST told the outpatient prescription volume in metformin last year marked 535.7 billion won growing 9.5 percent from the previous year.
The year 2019 showed a growth of 7.3 percent.
Metformin, a type of biguanides, is used vastly on Type 2 diabetic patients as a first-line treatment to control the blood sugar level.
Amid COVID-19 pandemic last year, the general prescription drug market fluctuated but the metformin market growth surged.

In last year, metformin single agent drug generated 77.3 billion won, showing a 4.1-percent growth compared to the year before.
The growth was increased steeply compared to 2.9 percent growth made in 2019.
Meanwhile, the metformin combination drug market had even higher growth.
The last year’s metformin combination drug prescription volume grew by 10.5 percent from the year before and made 458.4 billion won.
In 2019, the volume grew 8.2 percent compared to previous year.
The combination drug market surged by 96.8 percent throughout five years from 2015 making 232.9 billion won.
Currently, there are metformin drugs combined with dipeptidyl peptidase 4 (DPP-4) inhibitors, sulfonylureas, glitazone and sodium-glucose co-transporter-2 (SGLT2) inhibitors available in the South Korean market.
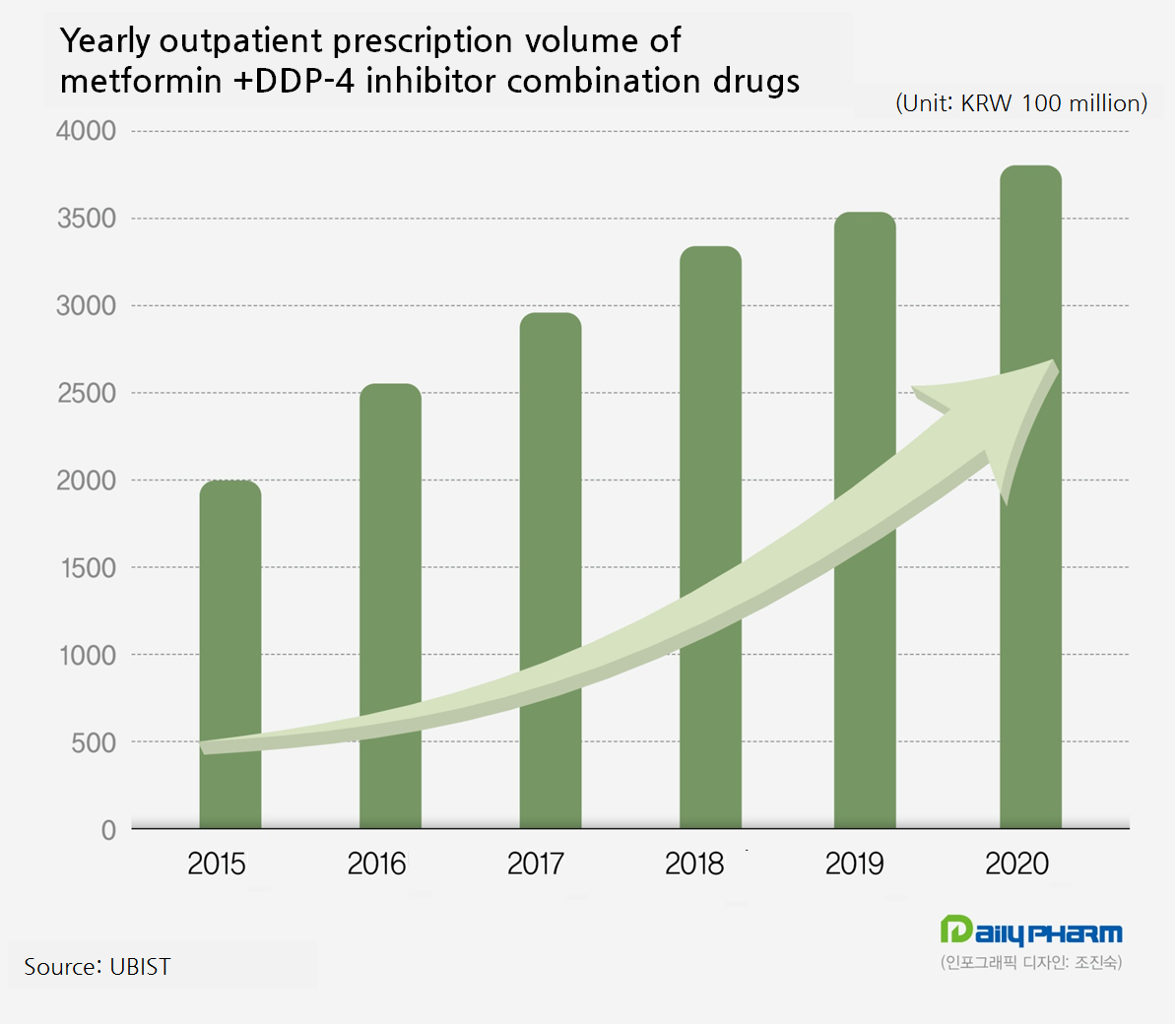
Among all combination drugs, DPP-4 inhibitor plus metformin drug market had a notable growth.
In last year, the DPP-4 inhibitor plus metformin drug prescription volume generated 380.5 billion won, growing by 5.8 percent compared to the year before.
In five years from 2015, the market expanded exponentially by 90.5 percent.
The DPP-4 inhibitor plus metformin drugs dominates 71.3 percent of the metformin combination drug market share.
Although metformin drug market suffered a detrimental hit by the impurity contamination, the prescription market firmly consolidated its ground.
South Korea’s Ministry of Food and Drug Safety (MFDS) ordered temporary manufacturing and sales suspension and prescription limitation on 31 metformin active pharmaceutical ingredient and complete products supplied in South Korea, as the ministry discovered unacceptable level of N-Nitrosodimethylamine (NDMA) carcinogen in the drugs.
The sales ban was imposed on JW Pharmaceutical’s Guardmet, Hanall Biopharma’s Glucodaun OR Tablet and Hutecs Korea Pharmaceutical’s Gluless-M.
The order was issued six months after the Singaporean health authority raised an issue of metformin impurity contamination.
In December 2019, Singapore’s Health Sciences Authority (HSA) investigated 46 metformin-contained items on sale and recalled three items as they found NDMA exceeding the acceptable daily intake level.
Since then, MFDS also started investigating metformin ingredients and complete products in South Korea.
The relevant industry was concerned when the South Korean health authority initiated the investigation as it could affect the metformin prescription market.
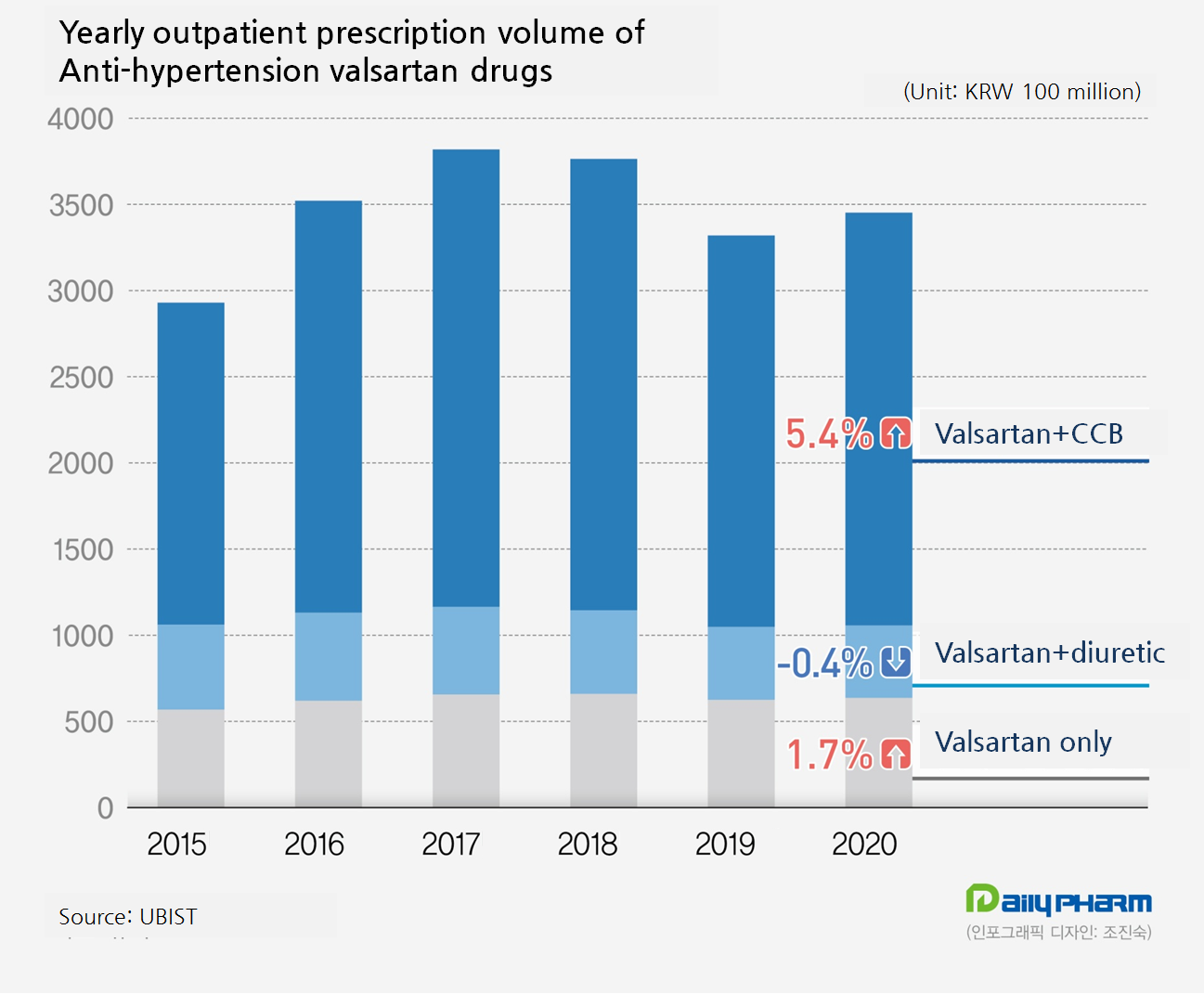
In fact, an anti-hypertension drug valsartan was also found with unacceptable level of impurity and the general market was impacted by the authority’s sales ban order.
In July and August 2018, MFDS banned the sales of 175 valsartan single agent drugs and combination drugs using ingredients found with NDMA.
Valsartan containing anti-hypertension treatments generated 381.9 billion won in 2017, but the volume continued to slip for two consecutive years in 2018 and 2019 at 376.4 billion won and 332 billion won, respectively.
But in last year, the volume increased by 4.0 percent compared to 2019 making a positive growth after three years.
Basically, the absence of alternative option has protected the drug’s credibility against the impurity incident.
Also the sales ban on metformin drugs was limited compared to the overall market.
MFDS reported the 31 items banned from sales generated production volume of 22.8 billion won in 2019.
It was only 6 percent of the overall production and import volume.
And other plenty of options were available, besides the banned items, for the prescription market to have avoided confusion.
The industry evaluates the previous experiences of impurity contamination in valsartan, ranitidine and nizatidine have taught the market such incident’s risk is insignificant.
When announcing the list of impurity-contaminated metformin items to be banned, MFDS concluded “The harmful risk on human body by long-term exposure to drugs found with unacceptable level of NDMA is miniscule.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









