- LOGIN
- MemberShip
- 2025-12-26 17:25:58
- Generic approvals soar again despite new stepped pricing
- by Chon, Seung-Hyun | translator Byun Kyung A | 2021-03-03 06:24:24
Despite being seemingly suppressed by the revised drug pricing system, the number of approved generic is soaring again.
Hundreds of Atozet and Januvia generics have entered the market and recorded the highest number generic approvals since May last year.
In the second half of last year, the pharmaceutical companies had a vacant gap in new approval as they have already had their generics approved before the pricing system revision.
However, the companies seem to jump into the generic approval competition, whenever a large generic market opens up, to take the advantageous pricing.
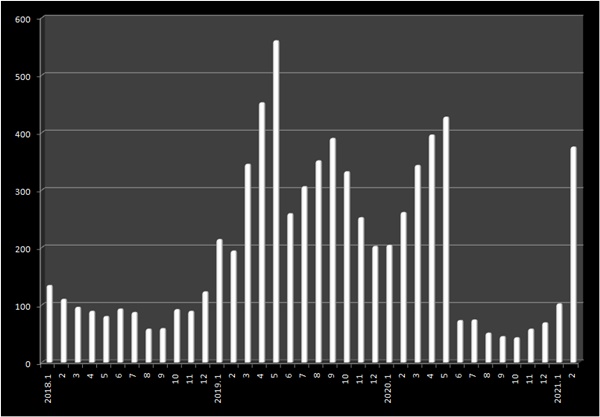
According to the Ministry of Food and Drug Safety (MFDS) on Mar.
2, 319 prescription generics have been authorized last month.
Compared to 102 generics approved in January, the number almost tripled.
The number of approved generic is surging compared to 50 items cleared from last June through December.
It has been nine months, since last May, the number of newly approved generics surpassed 300 in a month.
Apparently, 89 pharmaceutical companies received the South Korean health authority approval on total 256 items combining atorvastatin and ezetimibe.
The original combination drug is Atozet sold by MSD and Chong Kun Dang.
A group of generic makers all applied for approval after Jan.
11, when the original’s post-marketing surveillance period ended.
And 44 of sitagliptin generic versions of MSD’s original anti-diabetic Januvia were approved as well.
The number of generic approvals started rising rapidly from early 2019, and it slowed down in the latter half of last year.
Total 5,488 generics, or with monthly average of 323 items, were authorized from January through May 2019.
In a span of year in 2018, total 1,110 generics were approved with monthly average of 93 items.
The approval rate has tripled in just a year.
The government’s sign of tightening the generic regulation has eventually instigated the surge in generic approval.
Implemented from last July, the drug pricing system was revised to sustain the 53.55 percent of the original pricing before patent expiration for generics that individually conducted a bioequivalence test and use drug master file (DMF) submitted active ingredient.
Moreover, MFDS’ approach on regulatory changes in generic approval also encouraged the generic makers to rush for the approval.
The ministry warned of strengthening the regulation on joint bioequivalence test regulation by partially revising the Regulation on Pharmaceuticals Approval, Notification and Review in April 2019, which restricts the number of a manufacturer conducting a joint bioequivalence test.
Although the joint bioequivalence test regulation restriction never got passed due to the opposition by Regulatory Reform Committee at Government Policy Coordination Office, the application for generic approval soared immediately after the ministry disclosed the plan to restrict the bioequivalence test early last year.
However, the soaring number of generic approval application bounced back down after the revised drug pricing system was implemented.
The generic approval number, tallied up to be 427 as of last May, plunged to 73 in June.
For seven months, from July through December last year, 73, 51, 45, 43, 58 and 69 generics were approved, respectively, with monthly average of 58 shrinking down to 18 percent of the previous 18-month average.
The products that applied for the National Health Insurance (NHI) reimbursement before last May would receive the previous pricing benefits.
Technically, the generics that applied for the reimbursement from following June would be applied with the new lower pricing.
And it explains why the number of new generic approval applications dropped drastically from June.
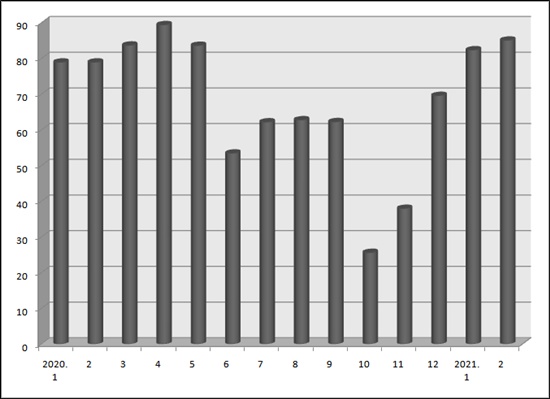
The recent surge in new generic approvals was led by the generic fully manufactured by consignment contract.
83 percent, or 84 out of 102 generics approved in last January were manufactured with consignment contract.
Also 85 percent, or 319 out of 375 generics approved in last month were the same.
From last October through December, only 26 percent, 38 percent and 70 percent of the prescription generics were respectively manufactured by consignment contract.
The majority of the generics would not be able to obtain high drug pricing without the individually conducted bioequivalence test, but they still joined the generic market.
In fact, entering the existing generic market late became meaningless with the reformed pricing system.
Due to the stepped pricing system, entering the market late would mean significantly low pricing.
The industry predicts the temporary exponential surge in generic approval would repeat in the future whenever a large new generic market opens up like Atozet’s.
Hundreds of Atozet generics were released due to huge marketability.
A pharmaceutical market research firm UBIST reported Atozet’s prescription volume last year reached 74.7 billion won making a year-on-year growth of 13.5 percent.
In two years from 2018, the volume rapidly surged by 60.6 percent from 45.4 billion won.
A pharmaceutical company associate commented, “Not many generic makers would join the already saturated market with the new stepped pricing system.
When a largely profitable original has its patent expire, a lot of generic makers would continue to try to launch their products at once to get the best pricing.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.









